C57BL/6 Mouse Pancreatic Islets
Cat.No.: CSC-C9291J
Species: Mouse
Source: Pancreatic Islet; Pancreas
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The pancreatic islet cells of C57BL/6 mice are isolated from the pancreas. The pancreas is divided into two parts: exocrine and endocrine. The Islets of Langerhans are the secretion site of islet hormones. There are four main types of pancreatic islet cells: α cells (glucagon), β cells (insulin), γ cells (somatostatin), and PP cells (pancreatic polypeptide). Mouse pancreatic islet cells in vitro are spindle-shaped or polygonal cells with the features of adhesive growth.
Mouse islet cells have important significance for diabetes research. The insulin secreted by β cells is one of the important hormones regulating blood glucose, while glucagon secreted by α cells can increase blood glucose when the body is hypoglycemic. Therefore, islet cells have important significance in the development, progression, and treatment of diabetes. By islet transplantation or β-cell injury, diabetes models can be constructed to study the pathogenesis and treatment of diabetes. C57BL/6 mouse islet cells are often used in islet transplantation experiments to assess post-transplant cell function and survival. The results show that the recipient mice can be independent of insulin after islet transplantation, and their blood glucose levels are significantly reduced. Islet cells also have an important role in metabolism studies. The function study of pancreatic islet cells is mainly aimed at the maintenance of blood glucose homeostasis. The secretion of insulin and glucagon and the metabolic role of C57BL/6 mouse islet cells are also studied.
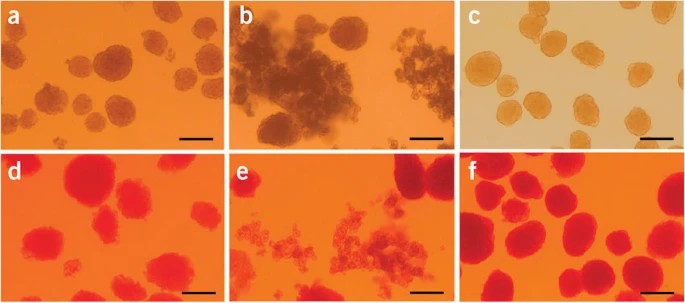 Fig. 1. The islets kept in cell culture medium (a,b,c) and the islets after dithizone staining (d,e,f). (a,d) Islets isolated through the present procedure before hand-picking. (b,e) Incompletely digested islets for troubleshooting. (c,f) Purified islets after hand-picking. The photos show the islets kept in the culture medium (top row) and the islets after dithizone staining (bottom row) (Li D S, Yuan Y H, et al., 2009).
Fig. 1. The islets kept in cell culture medium (a,b,c) and the islets after dithizone staining (d,e,f). (a,d) Islets isolated through the present procedure before hand-picking. (b,e) Incompletely digested islets for troubleshooting. (c,f) Purified islets after hand-picking. The photos show the islets kept in the culture medium (top row) and the islets after dithizone staining (bottom row) (Li D S, Yuan Y H, et al., 2009).
IMCs Suppressed Macrophage Migration Toward Allogeneic Islets In Vitro
Pancreatic islet transplantation (PITx) offers hope for type 1 diabetes patients, yet faces challenges due to intense post-transplant inflammation and innate immune responses. Previous studies have shown alloantigen-specific immunomodulatory cells (IMCs), grown ex vivo with anti-CD80/CD86 monoclonal antibodies, can induce tolerance post-liver transplantation. Forhioni et al. explored the potential of IMCs in mitigating inflammatory responses and protecting islets during PITx.
To evaluate IMCs' effect on macrophage migration to islets, an in vitro co-culture system mimicking allogeneic PITx was used. C57BL/6 pancreatic islets were paired with RAW264 macrophages, derived from BALB/c mice. The RAW264 cells were placed on a Transwell insert, allowing cell movement, with or without IMCs and C57BL/6 islets. After 24 hours, migrated F4/80+ cells in the lower wells were quantified using flow cytometry. RAW264 cells with only medium showed few macrophages (126.8 ± 123.1). IMCs alone slightly increased migration (1187 ± 605.1), while C57BL/6 islets alone majorly increased it (8212 ± 2157). Adding IMCs with islets notably decreased migration (4285 ± 1418, P < 0.05, Fig. 1B, C).
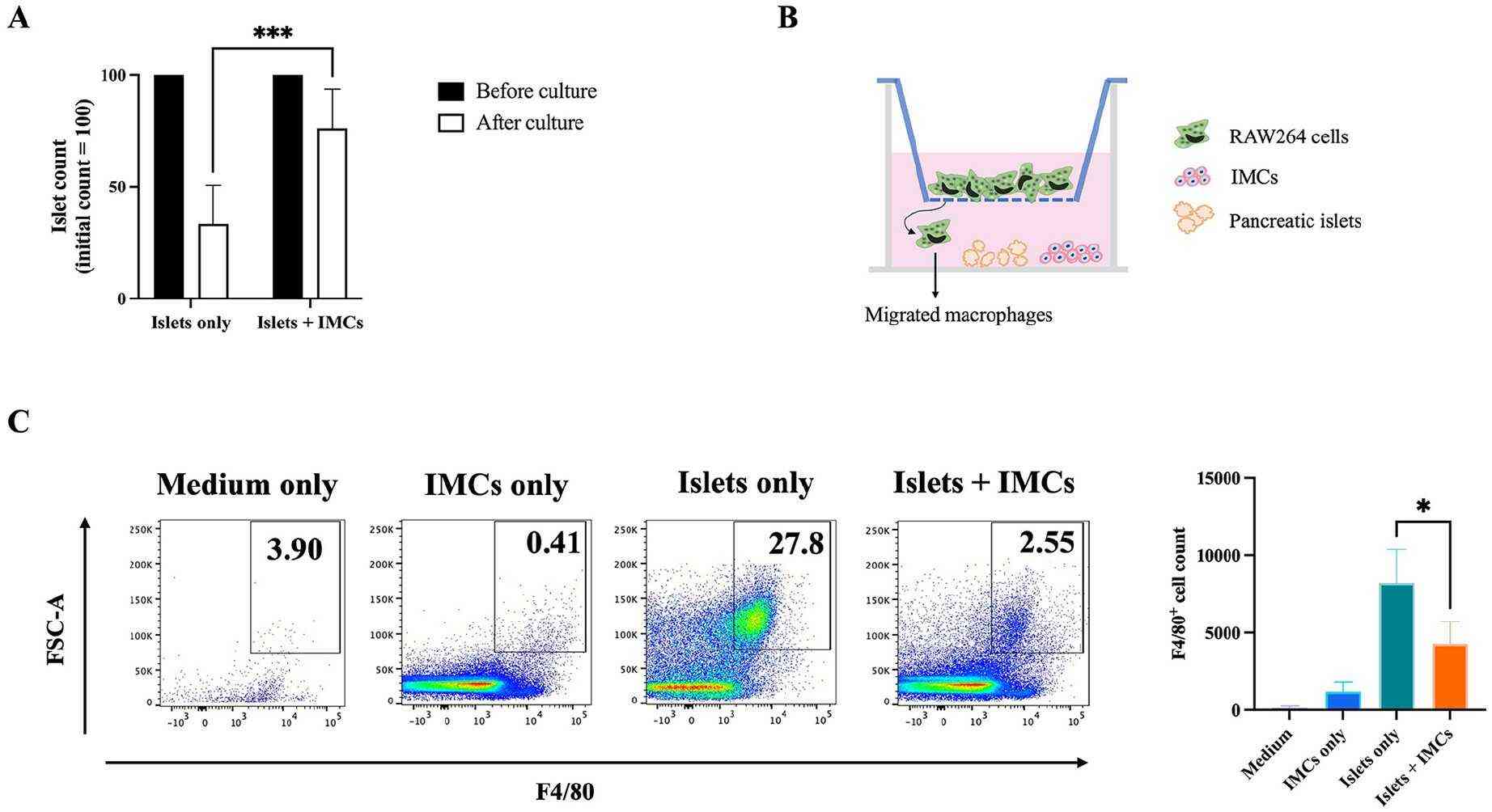 Fig. 1. Islet-macrophage co-culture with IMCs (Forhioni A, Watanabe M, et al., 2025).
Fig. 1. Islet-macrophage co-culture with IMCs (Forhioni A, Watanabe M, et al., 2025).
Chemical-Induced ER Stress Upregulates PLIN2 Expression in Pancreatic β Cells
Pancreatic β cell failure is pivotal in the progression from glucose intolerance to diabetes, with endoplasmic reticulum (ER) stress accelerating this process, particularly due to lipotoxicity and excessive insulin demand. ER stress leads to protein misfolding, triggering the unfolded protein response (UPR). Current studies highlight that perilipin 2 (PLIN2) affects lipid balance in the liver and is now identified as a modulator of both UPR and ER stress in pancreatic β cells.
In this study, Chen's team sought to determine the role of PLIN2 in modulating β cell function under ER stress conditions. They treated MIN6 cells with ER stress inducer tunicamycin (TM) and found that the treatment increased PLIN2 expression at both the mRNA and protein levels (Fig. 2A and B). The same treatment also stimulated PLIN2 protein expression (and a trend towards increased Plin2 mRNA expression) in C57BL/6 isolated mouse islets (Fig. 2C and D). In addition to TM, thapsigargin (THAPS), an ER stress inducer that works via different mechanisms25, also stimulated PLIN2 expression in MIN6 cells (Fig. 2E and F). The results suggest that increased ER stress per se is associated with upregulated PLIN2 expression.
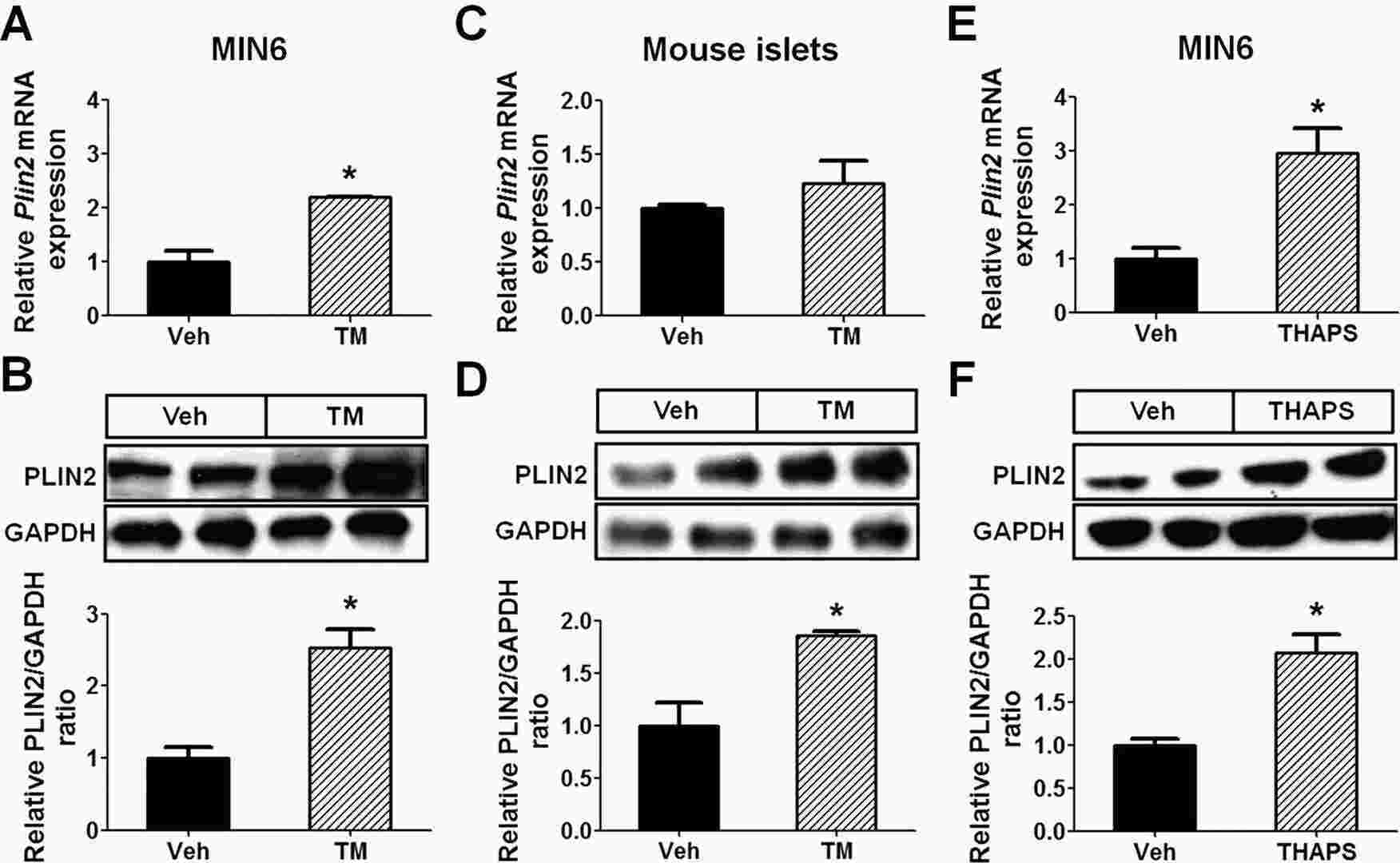 Fig. 2. ER stress upregulates PLIN2 expression in MIN6 cells and mouse pancreatic islets (Chen E, Tsai T H, et al., 2017).
Fig. 2. ER stress upregulates PLIN2 expression in MIN6 cells and mouse pancreatic islets (Chen E, Tsai T H, et al., 2017).
Ask a Question
Write your own review
- You May Also Need