C57BL/6 Mouse Lung Microvascular Endothelial Cells
Cat.No.: CSC-C4283X
Species: Mouse
Source: Lung
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The C57BL/6 mouse lung microvascular endothelial cells derive from pathogen-free mouse lung tissue and mainly originate from the pulmonary circulation's microvascular endothelium. These cells play essential roles in ensuring proper lung operations including gas exchange activities and immune system protection while supporting blood vessel development. The cells show distinct endothelial traits through an elongated or polygonal shape with tightly packed cell edges and visible cytoskeletal elements. In culture, these cells develop as single or multiple layers and create tight junctions with basement membranes that demonstrate their precise endothelial cell behavior. The cells demonstrate robust growth capabilities when cultured which allows them to stay alive from weeks up to months in specialized media thus proving their applicability for extended research periods.
These cells demonstrate remarkable specificity and culture properties which enable their wide application across cardiovascular disease research and drug screening and toxicity evaluation while also being key to gene knockout and transgenic model studies and inflammation and immune mechanism research in respiratory conditions such as asthma.
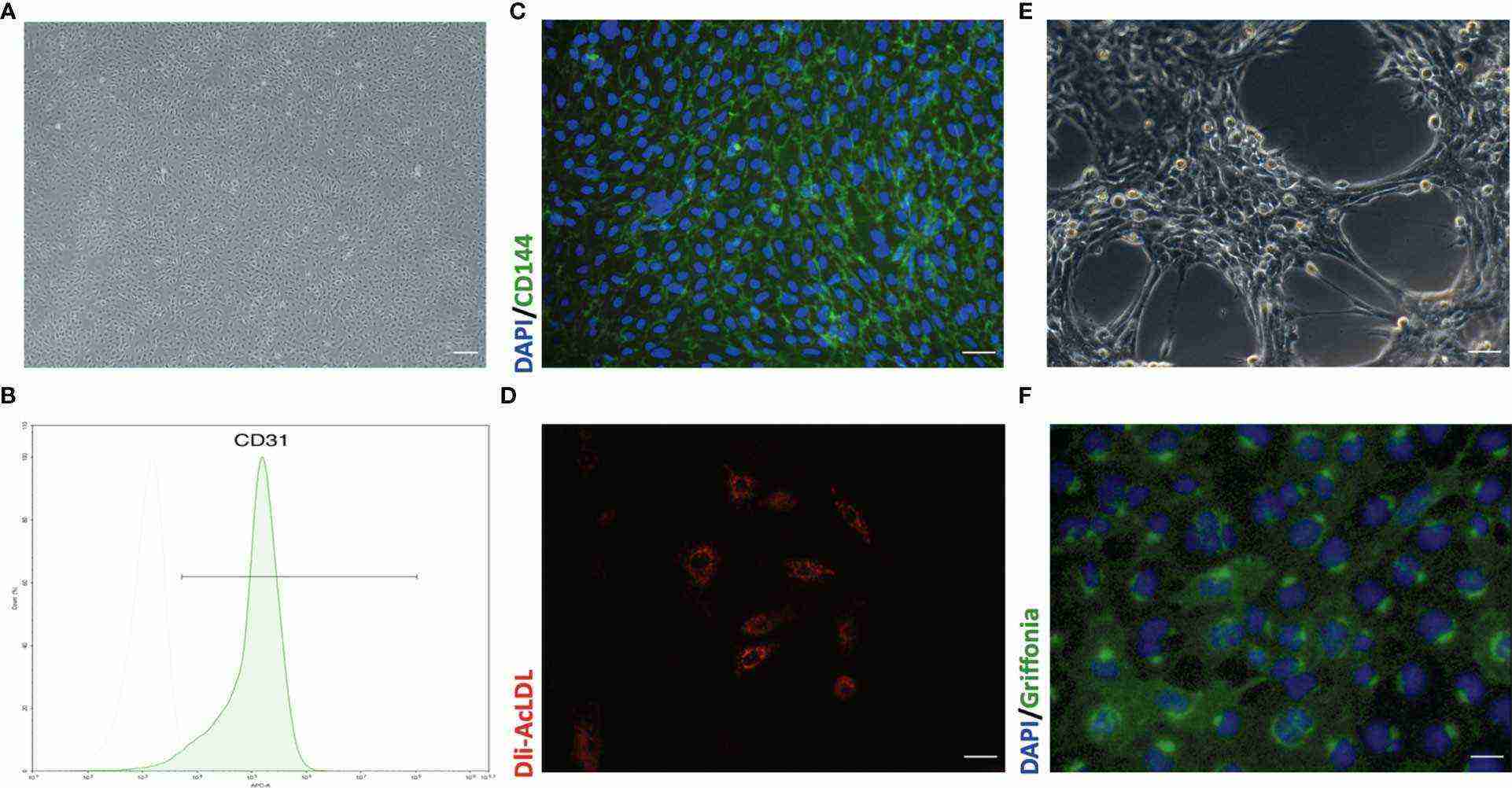 Fig. 1. Representative morphology and identification of primary mouse lung microvascular endothelial cells (Liu X, Xia F, et al., 2021).
Fig. 1. Representative morphology and identification of primary mouse lung microvascular endothelial cells (Liu X, Xia F, et al., 2021).
HDL from ARDS Patients Promotes the Dysfunction of Primary Cultured Pulmonary Microvascular Endothelial Cells
The abnormal systemic inflammatory response associated with septic-acute respiratory distress syndrome (ARDS) leads to acute lung injury (ALI) resulting in elevated morbidity and mortality rates. While high-density lipoprotein (HDL) normally defends against these health problems its dysfunction becomes a factor in organ failure during sepsis.
Yang's team compared HDL from septic-ARDS patients to normal controls to assess changes in composition and function. Septic-ARDS patients showed significant changes of HDL composition, accompanied with significantly decreased HDL-C. The similar plasma LPS levels in mice treated with both A-HDL and N-HDL suggest that A-HDL directly damages lung vascular endothelial cells thereby enhancing vulnerability to sepsis-induced dysfunction. Researchers assessed the effects of N-HDL and A-HDL on mouse lung microvascular endothelial cells from C57BL/6 mice by using human albumin-containing PBS as the control. A-HDL treatment led to a decrease in VE-cadherin levels while promoting VCAM1 expression but had no effect on ICAM1 levels (Fig. 1a). The Transwell assay with FITC-dextran tracer demonstrated that A-HDL increased endothelial permeability. A-HDL raised levels of inflammatory cytokines TNF-α and IL-6 as shown in Fig. 1b and c). A-HDL activated NF-κB signaling to boost P-p65/p65 ratios while N-HDL failed to do this (Fig. 1a). directly triggering pro-inflammatory signaling. HDL dysfunction leads to enhanced severity of sepsis-induced ALI/ARDS through direct damage to endothelial cells.
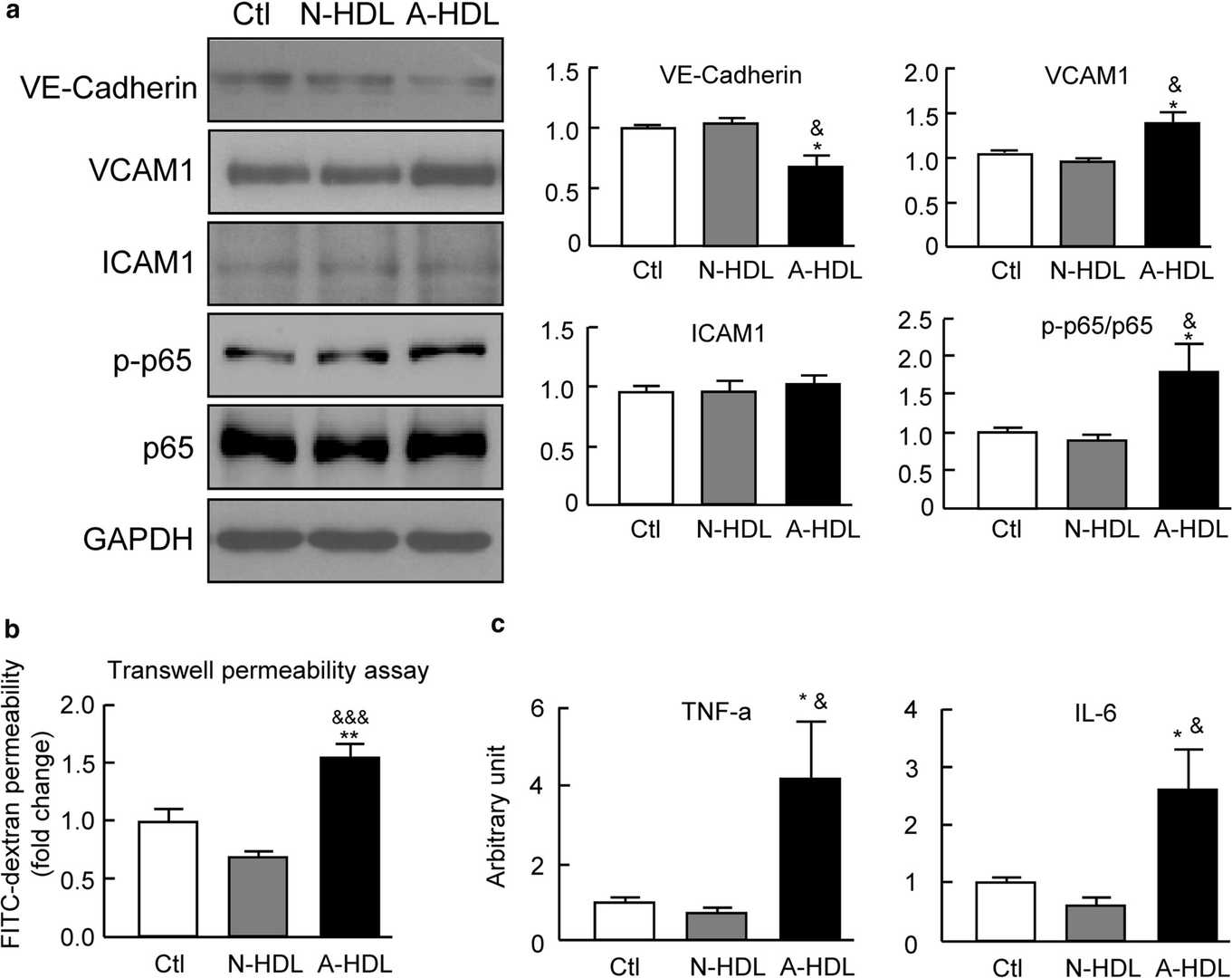 Fig. 1. The plasma HDL from ARDS patients promotes the dysfunction of primary pulmonary microvascular endothelial cells (Yang L, Liu S, et al., 2020).
Fig. 1. The plasma HDL from ARDS patients promotes the dysfunction of primary pulmonary microvascular endothelial cells (Yang L, Liu S, et al., 2020).
Aging Increases DNA Damage in Microvascular Endothelial Cells, as Shown by More 53BP1 foci
The risk of developing cardiovascular disease (CVD) increases substantially in older individuals because arterial function becomes impaired. The accumulation of DNA damage in arterial tissues appears to be a contributing factor although researchers have yet to fully explore how common and impactful this phenomenon is.
Bloom et al. used immunofluorescence detection of 53BP1 to assess DNA damage in lung microvascular endothelial cells derived from both young and old human and mouse donors to explore age-related DNA damage effects. The aging process increased both the percentage of endothelial cells with one or more 53BP1 foci and the number of 53BP1 foci per cell in human lung microvascular endothelial cells just like in large arteries (Fig. 2A-C). Research data reveals that mouse lung endothelial cells show an age-related increase in both the percentage of cells housing one or more 53BP1 foci (Fig. 1D and E) and the 53BP1 foci count per cell (Fig. 1D and F). These collected data demonstrate a direct link between aging and the proliferation of DNA damage in blood vessels.
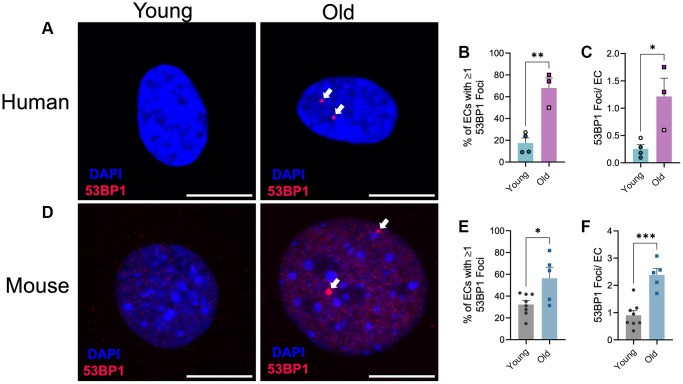 Fig. 2. Effect of aging on endothelial cell DNA damage (Bloom SI, Tucker JR, et al., 2023).
Fig. 2. Effect of aging on endothelial cell DNA damage (Bloom SI, Tucker JR, et al., 2023).
Ask a Question
Write your own review