C57BL/6 Mouse Liver Sinusoidal Endothelial Cells
Cat.No.: CSC-C4285X
Species: Mouse
Source: Liver
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
C57BL/6 mouse liver sinusoidal endothelial cells (LSECs) are a subpopulation of endothelial cells, located in the liver sinusoids, that line the hepatic microvasculature and help to regulate liver homeostasis. As an important barrier between the blood and liver parenchyma, LSECs are highly specialized and functionally different from other vascular endothelial cells. They have a fenestrated morphology, are non-organized in their basement membrane, and demonstrate high endocytic activity. As such, they are critical for taking up macromolecules, antigens, lipoproteins, and xenobiotics. LSECs are also important for modulating microcirculation in the liver, as well as immunologic tolerance and metabolic homeostasis.
C57BL/6 mouse LSECs are important for in vitro modeling of liver function. In addition to being used to model liver physiology, C57BL/6 mouse LSECs are important in biomedical research for a number of applications. They are an in vitro model to understand and characterize hepatic uptake, metabolism, and clearance in drug discovery and early phase pharmacokinetics/toxicology. Mouse LSECs are also used in the study of numerous liver pathophysiological states such as viral hepatitis, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, and hepatocellular carcinoma, as well as for angiogenesis, endothelial dysfunction, and liver regeneration in regenerative medicine and tissue engineering.
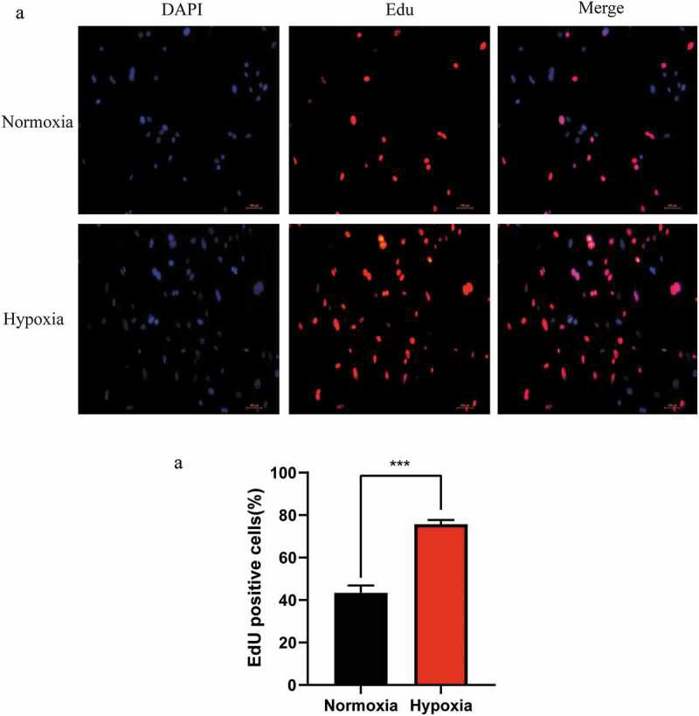
Hypoxia Promotes the Proliferation of Mouse Liver Sinusoidal Endothelial Cells: miRNA–mRNA Expression Analysis
In the early stage of liver regeneration (LR), hepatocytes and liver sinusoidal endothelial cells (LSECs) proliferate under the conditions of hypoxia. However, the role and molecular mechanism of LSECs under hypoxic conditions are still not fully understood. In this study, Qing’s team screened the miRNA–mRNA regulatory network involved in the proliferation of hypoxic LSECs.
First, they detected the proliferation of LSECs under hypoxic conditions to clarify the relationship between LSECs and liver regeneration in the early stage due to the hypoxic microenvironment. As shown in Figure1, EdU staining revealed more proliferation of LSECs in hypoxic conditions for 24 hours compared to normoxic conditions. These data suggest that hypoxia promotes LR through LSECs. Next, to explore the potential mechanism of hypoxia on LSEC proliferation, they performed high-throughput sequencing on LSECs under hypoxia (Group B) and normoxia (Group A), with n=3 per group. A total of 1,837 differentially expressed (DE) mRNAs (including 1,056 upregulated and 781 downregulated mRNAs) and 17 DE miRNAs (including 7 upregulated and 10 downregulated miRNAs) were identified. The target genes of DE miRNAs predicted by miRanda and TargetScan were intersected with DE mRNAs to obtain negatively correlated miRNA–mRNA pairs.
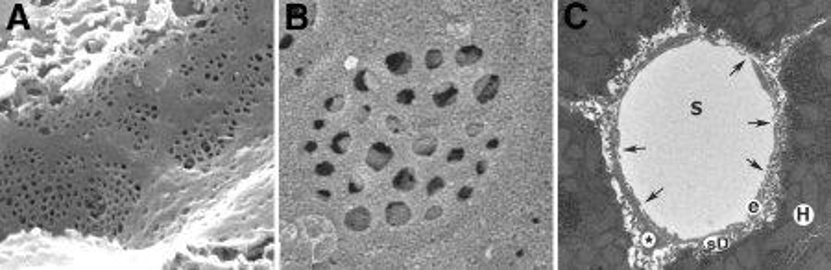
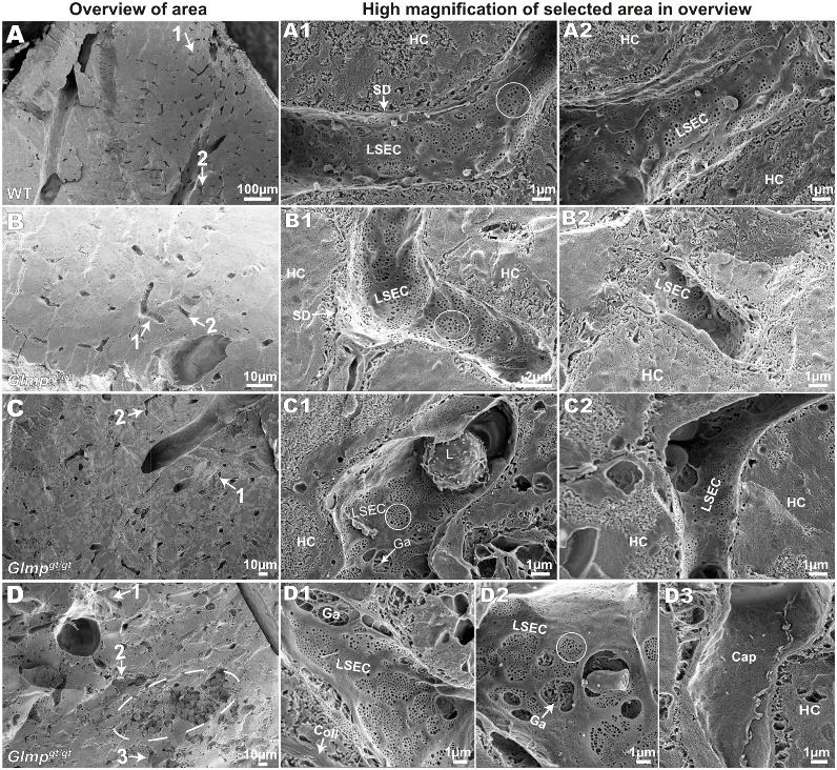
LSEC Morphology and Fenestration in Glmpgt/gt Livers in situ
Liver sinusoidal endothelial cells (LSECs) clear blood-borne waste through scavenger receptors, yet little is known about the role of LSECs in liver disease. Glmpgt/gt mice develop a chronic liver injury and fibrosis due to the deficiency in lysosomal membrane protein transport and represent an ideal model to study LSEC dysfunction. Here, Antwi et al. determined the role of Glmp in LSEC scavenger function and morphology.
To investigate LSEC morphology in Glmpgt/gt mice, liver tissues from 4-month-old (n=4) and 9–10-month-old (n=4) Glmpgt/gt male mice and WT controls (n=4, 4–6 months old) were scanned by EM. Tissues were sorted based on their median "% FITC-fluorescence area per liver tissue area". The LSECs in WT livers were highly fenestrated and possessed sieve plates with few gaps (>400 nm holes) (Fig. 2A1 and A2). In Glmpgt/gt, LSECs also retained fenestrations (circled) in the areas with fibrosis and inflammatory cell infiltration (Fig. 2B–D). Although, increased gaps were found near damaged hepatocytes and immune cells (Fig. 2C1, D1 and D2). Thick-walled non-fenestrated capillaries were also found in the areas with severe hepatocyte necrosis and destroyed sinusoids that possibly reflect neovascularization (Fig. 2D3).
Ask a Question
Write your own review