C57BL/6 Mouse Aortic Smooth Muscle Cells
Cat.No.: CSC-C4301X
Species: Mouse
Source: Aorta
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Aortic Vascular Smooth Muscle Cells are characterized by immunofluorescent cell staining with antibodies of α-smooth muscle actin and are negative for bacteria, yeast, fungi, and mycoplasma.
Cells can be expanded on a multiwell culture plate ready for experiments under the cell culture conditions specified by Creative Bioarray.
Repeated freezing and thawing of cells is not recommended.
Standard biochemical procedures performed with cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining, flow cytometry or generating cell derivatives for desired research applications.
C57BL/6 mouse aortic smooth muscle cells (mASMCs) are derived from the aorta of mice, particularly from the thoracic and descending aorta. The aorta is composed of three layers: the intima (endothelial cell layer), the media (smooth muscle cells and elastic fibers), and the adventitia (connective tissue). mASMCs are mainly found in the media of the aorta, and they are the main cell type for maintaining the elasticity and contractility of blood vessels. The cell morphology of them is diverse under in vitro cell culture conditions and can be spindle-shaped, irregular, triangular or fan-shaped. With the increase of passage number, the cells become more uniform gradually and display an elongated spindle shape, with rich cytoplasm and branch-like protrusions.
mASMCs are responsible for the execution of vascular contraction, and they can control the contraction and relaxation of blood vessels by changing the intracellular calcium ion concentration. In addition, mASMCs can secrete various cytokines, growth factors, and extracellular matrix, and they are also involved in vascular wall remodeling and repair. In addition, they can express various adhesion molecules (such as ICAM-1, VCAM-1) under inflammatory stimulation and can also promote leukocyte infiltration and have a role in the development of atherosclerosis. Therefore, mASMCs are widely used in research on the mechanisms of diseases such as atherosclerosis, abdominal aortic aneurysms, hypertension, and vascular remodeling.
TMAO Increases AngII-Induced Senescence and ROS Accumulation In Vitro
The pathology of abdominal aortic aneurysm (AAA) involves degradation of the extracellular matrix, oxidative stress, vascular inflammation, and smooth muscle cell senescence. Trimethylamine N-oxide (TMAO) is a gut microbial metabolite associated with CVD and has been shown to induce vascular inflammation and SMC senescence. The link between TMAO and AAA development has not yet been identified. Hu's team aims to investigate the association between TMAO and AAA formation and explore the underlying mechanisms.
In order to investigate the effects of TMAO on mouse aortic smooth muscle cells (SMCs), SMCs were incubated with AngII and/or TMAO before the detection of SA-β activity. SA-β staining revealed that AngII significantly increased SA-β activity, and TMAO treatment further increased the effect (Fig. 1A, C). Notably, as shown in Figure 1B, TMAO further exacerbated the AngII-induced ROS accumulation in SMCs. In contrast, TMAO treatment alone did not significantly affect SMCs (Fig. 1B, D). The expression of AAA-related MMP and senescence-related markers after TMAO treatment was further analyzed. TMAO further increased the AngII-induced expression of MMP2, MMP9, p16, and p21 in a dose-dependent manner (Fig. 1E–F). Taken together, the results suggested that TMAO exacerbates AngII-induced cellular senescence and ROS accumulation, which might be a possible mechanism by which TMAO promotes AAA formation.
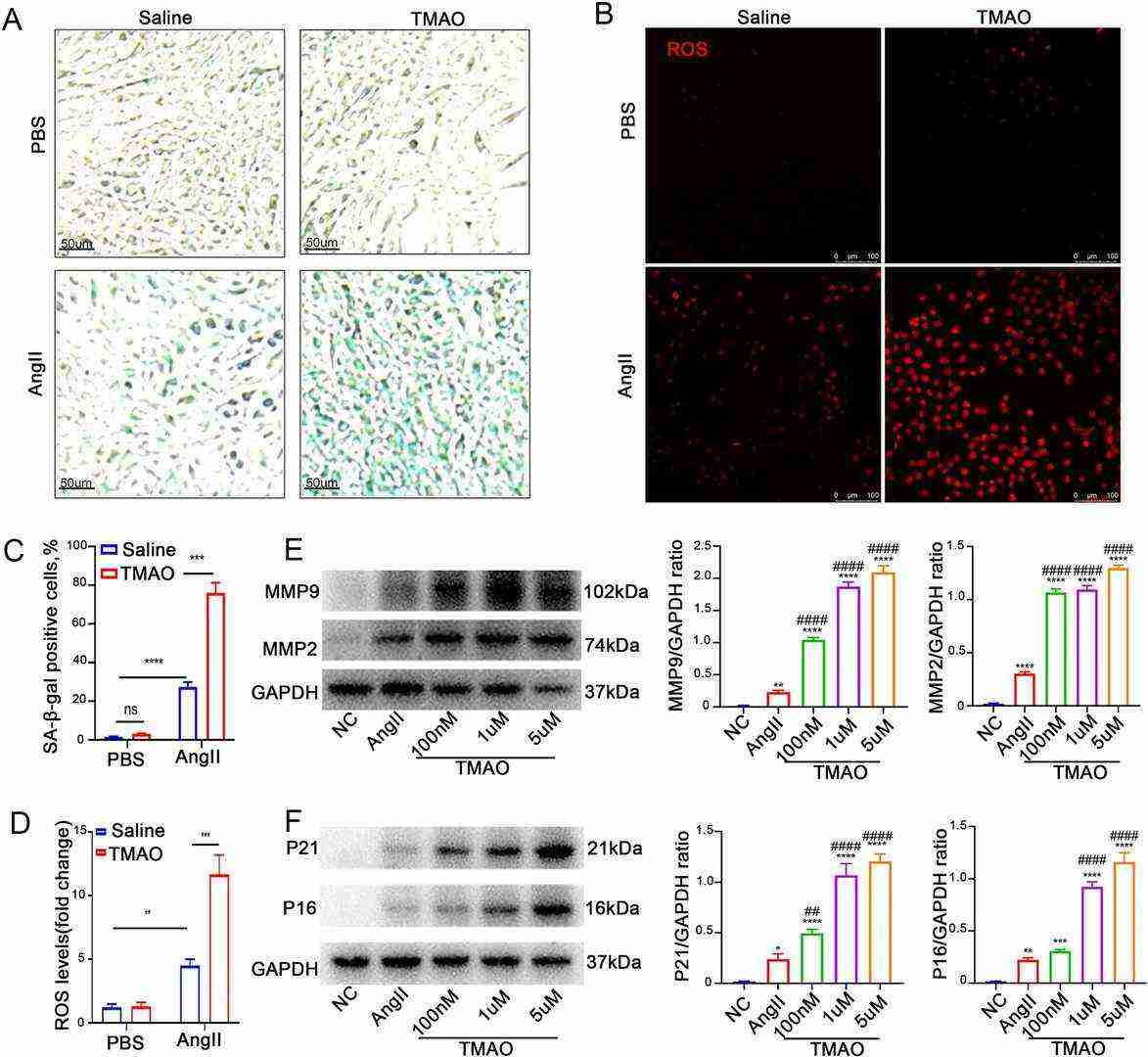 Fig. 1. TMAO increases AngII-induced cellular senescence and ROS accumulation in vitro (Hu J X, Xu J M, et al., 2022).
Fig. 1. TMAO increases AngII-induced cellular senescence and ROS accumulation in vitro (Hu J X, Xu J M, et al., 2022).
FBLN7 in VSMCs is Associated with Vascular Remodeling
Arterial remodeling is a critical pathophysiological process of diseases like hypertension. Fibulin-7 (FBLN7) is an adhesion protein, and its function in vascular remodeling is still unclear. Here, Yao's team try to clarify whether FBLN7 regulates vascular remodeling and its mechanism.
To test whether FBLN7 was involved in vascular remodeling, they analyzed high-throughput sequencing data from two models of abdominal aortic aneurysm and hypertensive vascular remodeling from the GEO database (Fig. 2A). In the AAA model, FBLN7 expression was increased at 2 and 4 weeks after Ang II infusion than in the control group (Fig. 2B). Next, they analyzed the FBLN7 expression in vascular wall cells by single-cell sequencing data from human aortic tissue samples (Fig. 2F). The result showed that FBLN7 was highly expressed in smooth muscle cells, fibroblasts, and pericytes. They also verified the protein expression of FBLN7 in mouse aortic smooth muscle cells (MASMCs), endothelial cells (MAECs), fibroblasts (MAFs), and peritoneal macrophages (MPMs) by Western blot analysis and found that FBLN7 expression was high in MASMCs (Fig. 2G). Vascular smooth muscle cells (VSMCs) play an essential role in vascular remodeling; therefore, they tested the MASMCs stimulated by Ang II and found that FBLN7 expression was increased after Ang II treatment. Immunohistochemical staining and immunofluorescence staining further confirmed the high expression of FBLN7 in the media after Ang II infusion and colocalization of FBLN7 with the VSMC marker α-SMA (Figs. 2H and I). These results indicate a crucial role of FBLN7 in hypertension-induced vascular remodeling.
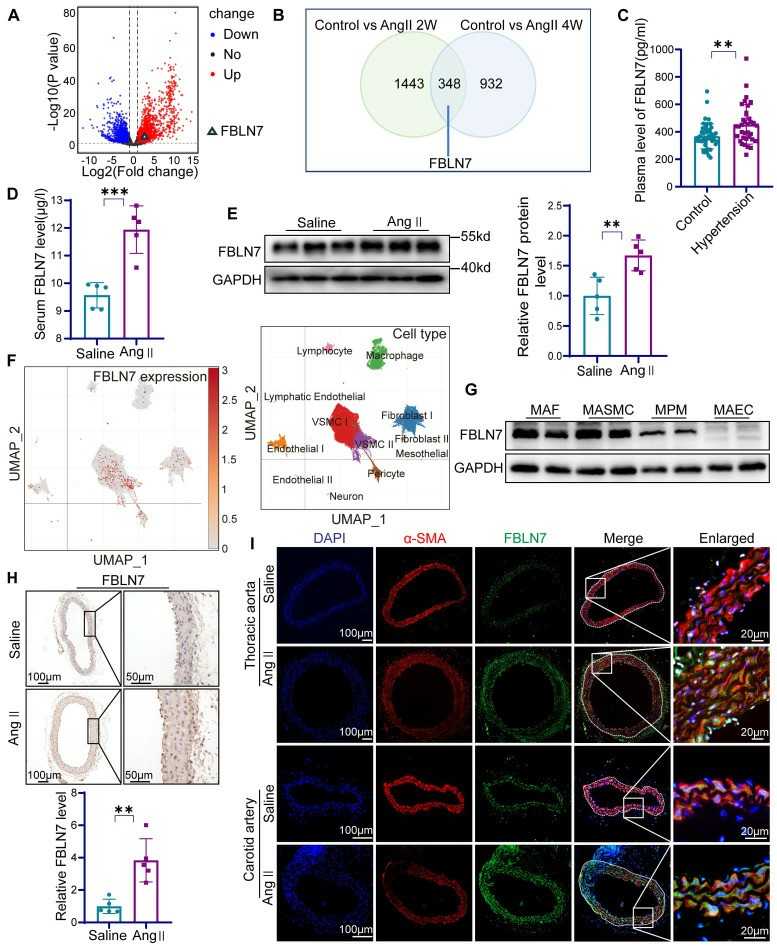 Fig. 2. The expression of FBLN7 is increased in vascular remodeling (Yao G Q, Zheng X H, et al., 2024).
Fig. 2. The expression of FBLN7 is increased in vascular remodeling (Yao G Q, Zheng X H, et al., 2024).
Ask a Question
Write your own review