BALB/c Mouse Brain Microvascular Endothelial Cells
Cat.No.: CSC-C4346X
Species: Mouse
Source: Brain
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
BALB/c mouse brain microvascular endothelial cells (BMECs) originate from the microvascular endothelium within the mouse brain and are key components of the blood-brain barrier (BBB). As an essential component of the blood-brain barrier, BMECs have extremely selective permeability, allowing nutrients and oxygen in the blood to diffuse smoothly while preventing harmful substances and pathogens from entering the brain, thereby providing strong external support for the normal physiological function of the central nervous system. They are also actively involved in the transport of nutrients into the brain and the removal of metabolic waste, and play an irreplaceable role in maintaining the normal physiological function of the brain. In addition, BMECs can secrete cytokines, chemokines and other signal molecules and are involved in intercellular signal transduction. They can also regulate various physiological processes such as immune response and inflammation. In important physiological and pathological processes such as brain development, injury repair, and angiogenesis, BMECs also play a vital role. It can promote new blood vessel formation and repair damaged blood vessels.
The BMECs cell line has extensive applications in scientific research. On the one hand, this cell line can be used as an important model for studying the structure and function of the blood-brain barrier, drug permeability of the blood-brain barrier and even screening and evaluation of drugs for brain penetration ability. On the other hand, it can be used to study the pathogenesis and treatment of various neurological diseases, including cerebrovascular and neurodegenerative diseases. In addition, this cell line can also be used to study the interaction between brain microvascular endothelial cells and other cells such as neurons and immune cells, and the specific functions of this interaction in physiological and pathological processes.
Development of the Mouse BBB Transwell and Microfluidic BBB Chip
Organ-on-a-chip technology, which mimics in vivo vascular dynamics, has shown promise for studying organ-specific vascular beds. However, its advantages over traditional in vitro models in identifying vascular-targeted drug delivery systems (DDS) are still not fully explored. Choi et al. aims to demonstrate the reliability and efficacy of organ-on-a-chip in screening efficient DDS by comparing it with a conventional transwell, both designed to simulate the blood–brain barrier (BBB).
To compare the efficacy of traditional transwell and microfluidic OoC approaches for screening functional shuttle ligands, they created BBB transwell and BBB Chip systems using primary BBB cells from BALB/c mice. BALB/c mouse brain microvascular endothelial cells were cultured either on a transwell insert's PET membrane or in the OoC device's lower vascular channel. To mimic the BBB, a mixture of mACs and mPCs was cocultured with an mBEC monolayer in both systems. Within 24 h, each model achieved a confluent mBEC layer, which was maintained in growth media until day 3. On day 3, the models were fed with a maturation medium to enhance BBB attributes (Fig. 1a). The highest TEER value was observed after 1 day of maturation medium exposure and remained stable for 4 days. After 1 day of maturation, the BBB Chips were subjected to 100 μL/h flow for 24 h. On day 5, expression of BBB marker genes significantly increased compared to day 3, indicating mature BBB cultures. mBECs in the BBB Chip were elongated and aligned along the fluid flow direction (Fig. 1b). The established mouse BBB models clearly expressed junctional proteins like ZO-1 and Claudin-5 under confocal immunofluorescence microscopy (Fig. 1c and 1d). To verify model integrity, paracellular permeability was measured using 10 kDa dextran (Fig. 1e). Both models showed significantly lower dextran permeability than controls.
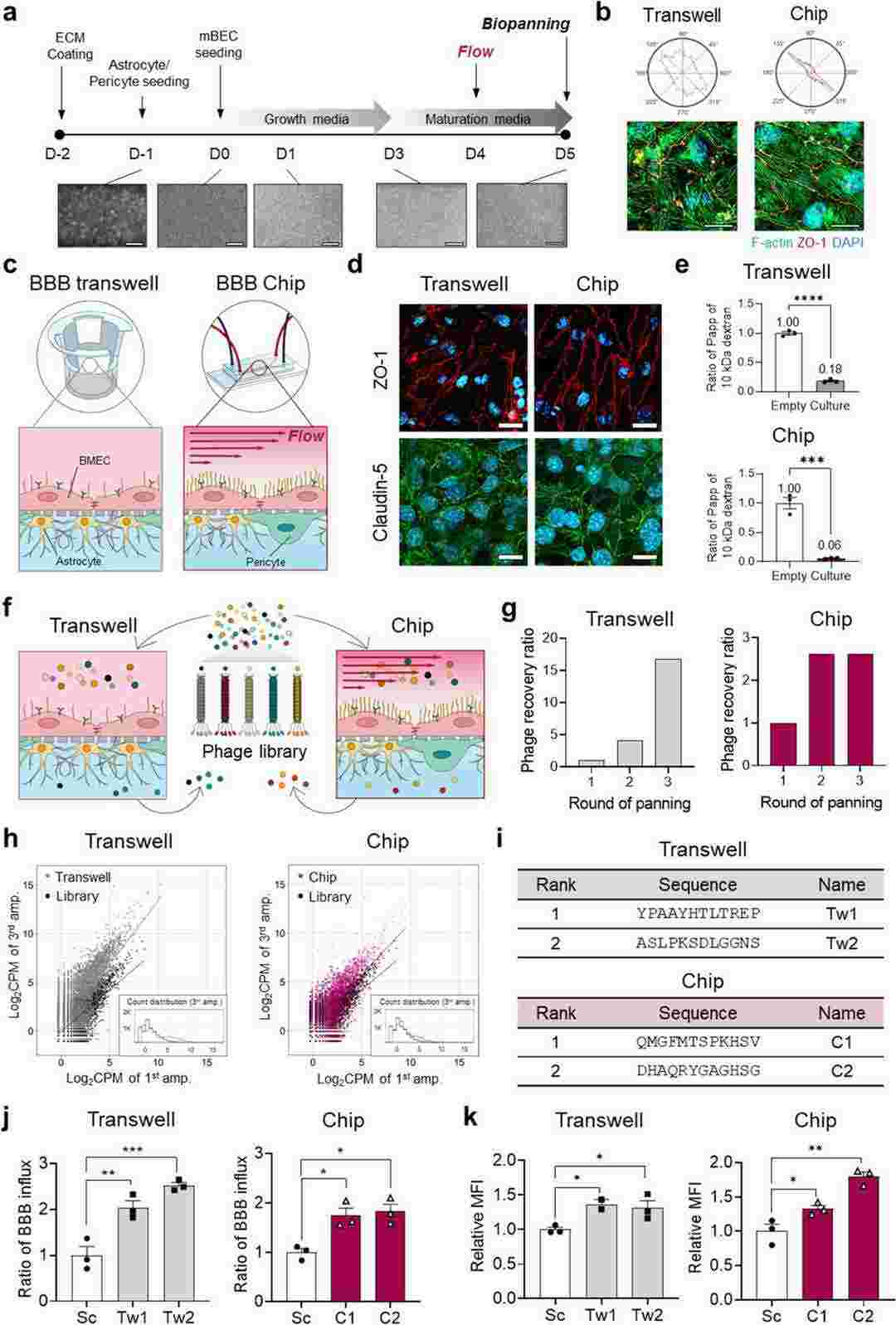 Fig. 1. Reconstruction of the mouse BBB in a transwell and a microfluidic device and biopanning to identify BBB nanoshuttle peptides (Choi J, Kim K, et al., 2024).
Fig. 1. Reconstruction of the mouse BBB in a transwell and a microfluidic device and biopanning to identify BBB nanoshuttle peptides (Choi J, Kim K, et al., 2024).
Effect of Tryptase on Mouse Brain Microvascular Endothelial Cells via Protease-Activated Receptor 2
Mast cells (MCs) are key players in brain injury, capable of disrupting the blood–brain barrier (BBB), yet the exact mechanism remains unclear. Tryptase, the most abundant MC secretory product, activates protease-activated receptor 2 (PAR-2), which is highly expressed in brain microvascular endothelial cells. These cells form the BBB and are crucial for its function and integrity. Zhou's team aim to explore the effects of tryptase on mouse brain microvascular endothelial cells and its potential mechanisms.
The Cell Counting Kit-8 (CCK-8) assay was used to evaluate the toxic effects of tryptase and FS (a PAR-2 inhibitor) on mouse brain microvascular endothelial cell. The mouse brain microvascular endothelial cells were divided into eight groups, including different concentrations of tryptase (0.001, 0.01, 0.1, 1 and 10 μg/mL), FS (400 μM, 800 μM) and control group. Then cell viability was measured by the CCK-8 assay after incubating 24 h. Their results indicate that tryptase (1 μg /mL) and 400 uM FS exerted no obvious toxic effects on mouse brain microvascular endothelial cell (Fig. 2). As tryptase is the natural agonist of PAR-2, they examined the influence of tryptase on the expression of PAR-2 by western blotting and immunofluorescence. As shown in Fig. 3a, b, tryptase could upregulate the protein level of PAR-2. However, pre-treatment with FS (a PAR-2 inhibitor) resulted in decreased expression of PAR-2 compared with the control group.
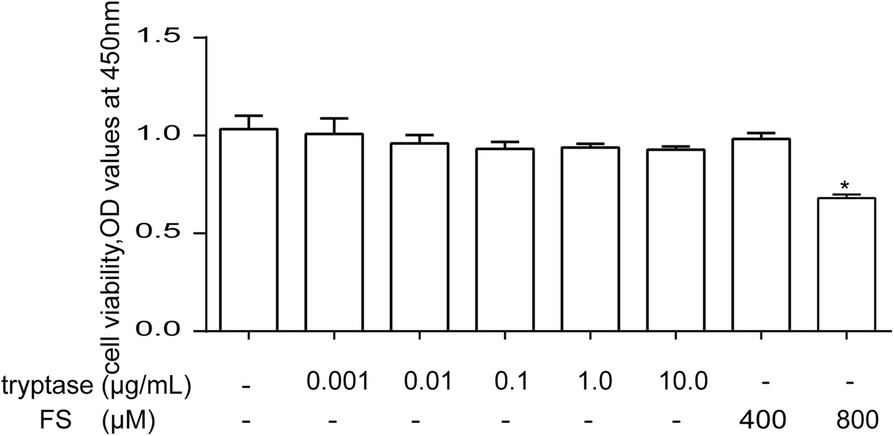 Fig. 2. The effects of tryptase and FS on cell viability in mouse brain microvascular endothelial cell (Zhou Q, Wang Y W, et al., 2018).
Fig. 2. The effects of tryptase and FS on cell viability in mouse brain microvascular endothelial cell (Zhou Q, Wang Y W, et al., 2018).
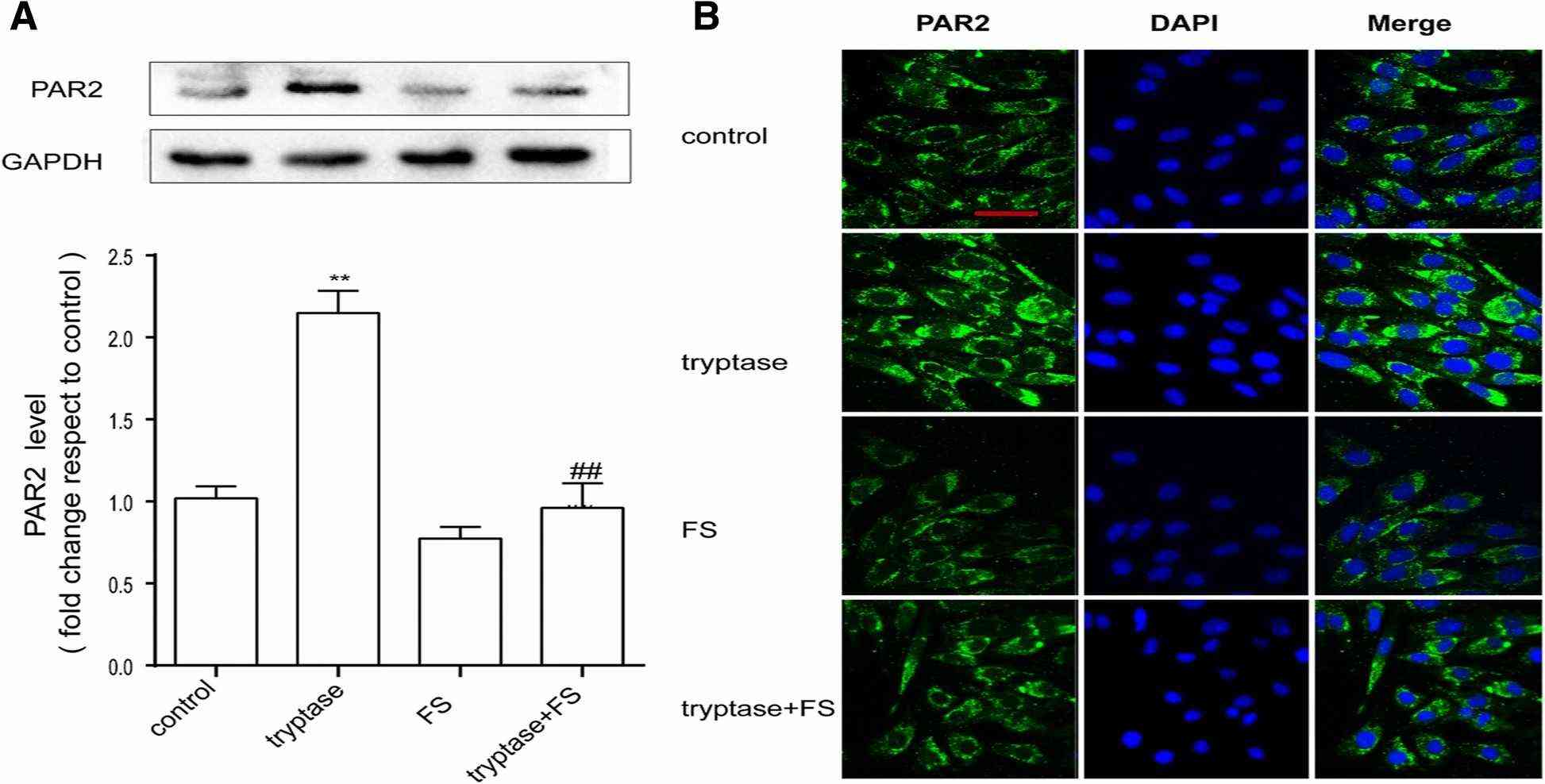 Fig. 3. Tryptase can increase PAR-2 levels (Zhou Q, Wang Y W, et al., 2018).
Fig. 3. Tryptase can increase PAR-2 levels (Zhou Q, Wang Y W, et al., 2018).
Ask a Question
Write your own review