
Immortalized Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes-hTERT
Cat.No.: CSC-I2074Z
Species: Homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Note: Never can cells be kept at -20°C.
Fibroblast-like synoviocytes (FLS) are mesenchymal origin cells that reside within the synovium. FLS display many characteristics common with fibroblasts, such as expression of several types of collagens and protein vimentin, a part of cytoskeletal filament. Once activated, FLS are thought to acquire a myofibroblast-like phenotype, driving fibrogenesis through excessive extracellular matrix component deposition and an enhanced contractile function. These cells play a crucial role in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis. Therefore, Human FLS are an important model for understanding articular function in degenerative and inflammatory joint diseases.
Human fibroblast-like synoviocytes from patients with rheumatoid arthritis have been used in research to evaluate:
- Signaling pathways associated with joint inflammation and the development of and rheumatoid arthritis.
- The contribution of EBV to inflammation in non-resolving rheumatoid arthritis by inducing IL-6 production by synoviocytes
- Anti-inflammatory and antirheumatic activity of various compounds.
- Anti-inflammatory effects of low level light therapy
- Pathogenic factor, immunopathological mechanisms and signal transduction pathways contributing to joint inflammation in rheumatoid arthritis.
- The role of estrogen signaling in enhancing inflammation.
- Involvement of capsid proteins of parvorvirus B19 in activating synoviocyte migration and induction of the inflammatory response.
- The role of human endogenous retroviruses (HERVs) in development of rheumatoid arthritis, with the suggestion that activated expression of different forms of HERV contribute to development of rheumatoid arthritis symptoms by different mechanisms.
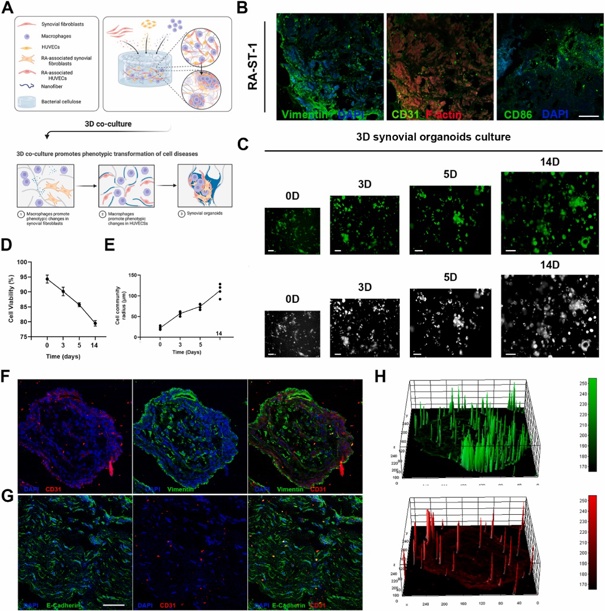
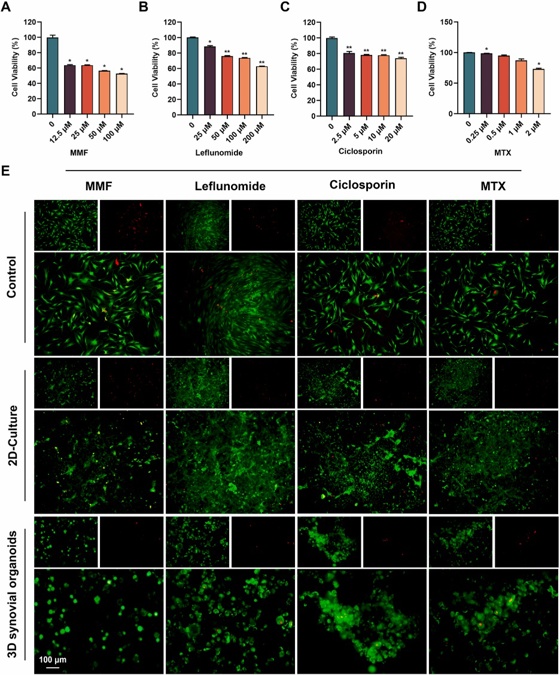
Constructing A 3D Co-Culture In Vitro Synovial Tissue Model for Rheumatoid Arthritis Research
The development and exploration of highly effective drugs for rheumatoid arthritis remains urgent. However, existing disease research models are unable to meet the rapid development needs of high-throughput drug screening. In this study, bacterial cellulose (BC) simulating the structure of extracellular matrix was used as a 3D culture platform, and THP-1-derived M1 macrophages, representing the inflammatory component, human umbilical vein endothelial cells (HUVECs), simulating the vascular component, and rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs), embodying the synovial pathology, were co-cultured to simulate the pathological microenvironment in RA synovial tissues, and synovial organoids were constructed.
Compared to planar cultures and 2D co-cultures, 3D synovial organoids not only exhibit a broader range of transcriptomic features characteristic of rheumatoid arthritis but also demonstrate increased drug resistance, likely due to the more complex and physiologically relevant cell-cell and cell-matrix interactions present in 3D environments. This model offers a promising path for personalized treatment, accelerating precision medicine in rheumatology.
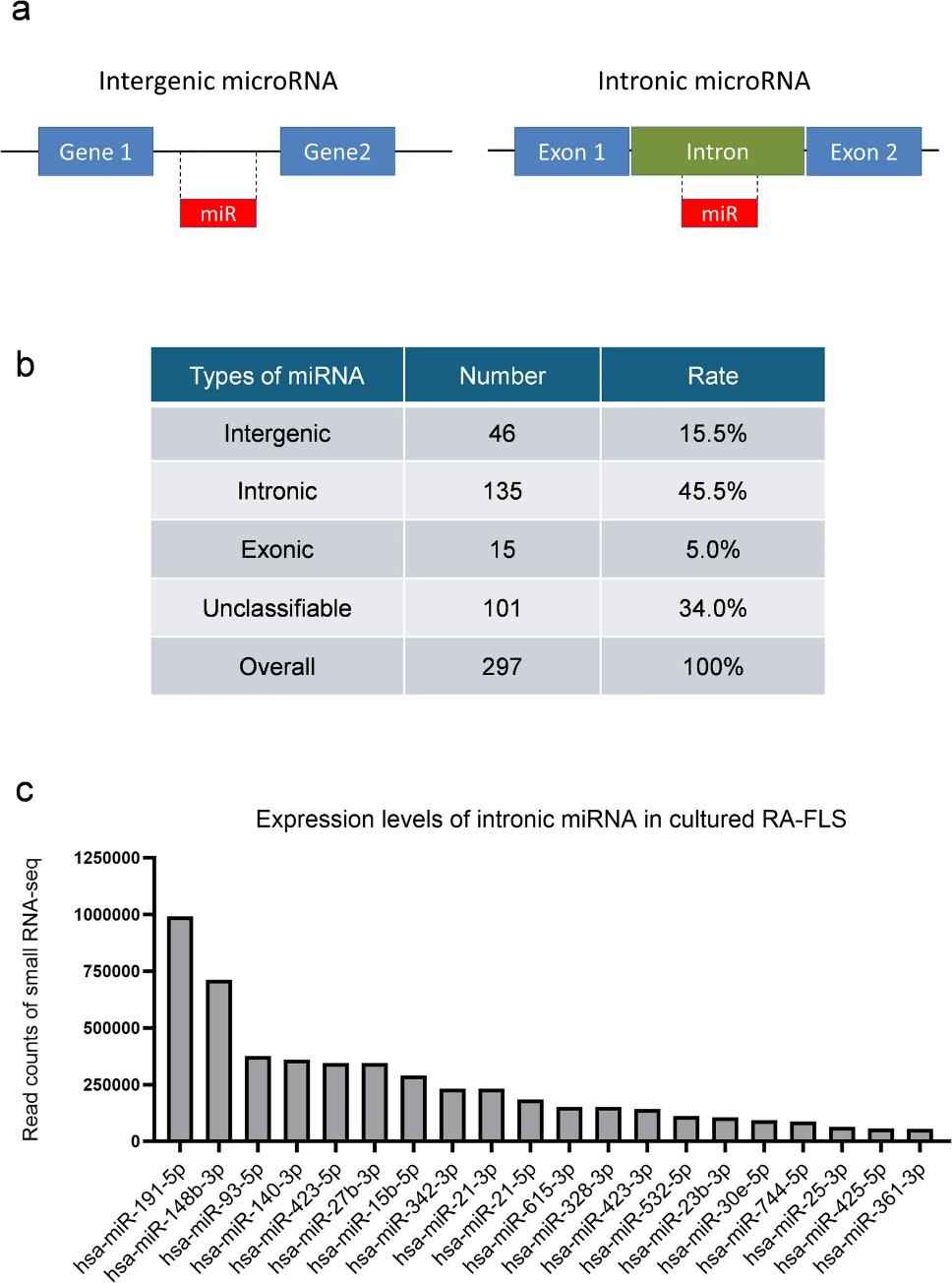
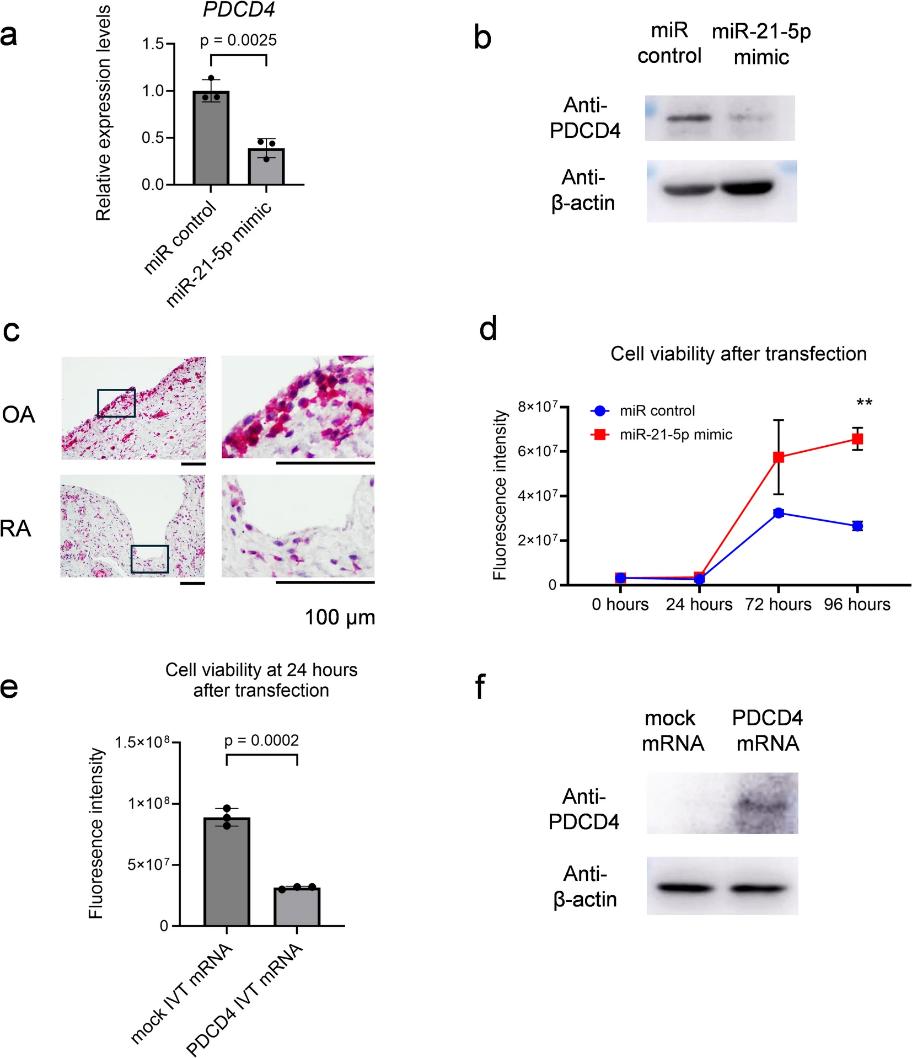
Hsa-miR-21-5p Affects Multiple Pathogenic Factors Associated With Fibroblast-Like Synoviocytes in Rheumatoid Arthritis
Fibroblast-like synoviocytes (FLS) contribute significantly to the pathogenesis of rheumatoid arthritis (RA), particularly through their roles in synovitis and joint destruction. The microRNAs (miRNAs) are short non-coding RNA that regulate gene expression post-transcriptionally. Although more than 2000 human miRNAs are registered, comprehensive analyses of miRNA expression in FLS are limited. Herein, we investigated the relationship between miRNAs and FLS.
Next-generation sequencing (small RNA-seq) was performed on primary cultured FLS derived from patients with RA to analyze the mature miRNA expression pattern. MiRNA mimics were transfected into FLS and an immortalized synovial fibroblast cell line, and validated using conventional RNA-seq. Out of 2861 mature miRNAs, 297 mature miRNAs were detected and intronic miRNAs were predominated. Notably, hsa-miR-21-5p was abundantly expressed and its expression was enhanced by IL-6 stimulation. MiR-21-5p mimic led to enhanced cell proliferation. These data suggest that hsa-miR-21-5p in FLS may exacerbate the pathophysiology of rheumatoid synovitis by promoting FLS proliferation, highlighting its potential as a therapeutic target in RA.
Our dedicated technical support team provides extensive assistance with experimental setup, culture optimization, and troubleshooting to help you maximize the value of these cells in your research endeavors.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

