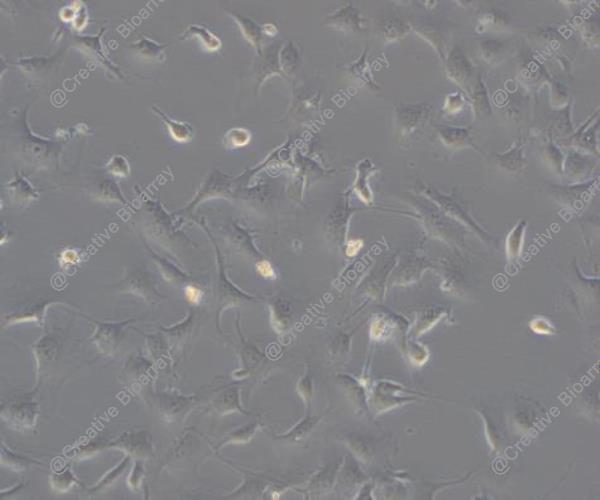
Immortalized Human Pericytes
Cat.No.: CSC-C12030Z
Species: Homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Note: Never can cells be kept at -20°C.
Pericytes are non-endothelial cells that reside on the inner layer of blood vessel walls, typically located on the basement membrane of capillaries, where they form tight interactions with endothelial cells. Pericytes can be found in various body tissues including the central nervous system, peripheral nervous system, skeletal muscle, adipose tissue, lungs and skin. Pericytes maintain and establish the blood-brain barrier throughout the central nervous system. Within the pulmonary system, these cells work together with endothelial cells to maintain both the structure and functionality of the blood vessels. Scientists create immortalized human pericytes from primary cells by genetically altering them using SV40T or hTERT transfection methods. The cells demonstrate a stellate or spindle form with multiple projections which establish tight connections to endothelial cells. When viewed through a microscope immortalized pericytes show large nuclei and unevenly distributed chromatin along with an abundant cytoplasm containing rich rough endoplasmic reticulum. Furthermore, the cells demonstrate multiple pericyte markers when stained for immunofluorescence which includes PDGFRβ, NG2, CD44, CD146, CD90, and CD73.
Pericytes play a crucial role in building and maintaining the blood-brain barrier which makes them essential components for developing in vitro blood-brain barrier models. The essential functions of these cells in angiogenesis and vascular homeostasis make them fundamental for examining cellular dynamics during the angiogenesis process. Additionally, pericytes potentially affect tumor metastasis and researchers use them to study how tumor cells interact with pericytes.
HBMVEC Co-Culture with Human Pericytes Increased Barrier Properties
The blood-brain barrier (BBB) limits drug access to brain tumors due to tight junctions (TJs) in endothelial cells. Tumor Treating Fields (TTFields) have been shown to increase BBB permeability in murine models. In this study, Salvador et al. established a 3D co-culture model of the BBB using human brain microvascular endothelial cells (HBMVEC) and pericytes.
They first examined the effects of 100 kHz TTFields on HBMVEC monocultures. After 72 h of treatment, immunofluorescence staining showed a slight change in claudin-5 localization (Fig. 1A). However, compared to cerebEND cells, which exhibited distinct claudin-5 delocalization from cell borders to the cytoplasm, HBMVEC showed weaker staining and lower claudin-5 expression in both treated and untreated conditions (Fig. 1B). They then co-cultured HBMVEC with immortalized human pericytes, which are known to enhance BBB characteristics, including tight junction protein expression. Western blot analysis revealed that pericytes increased ZO-1 and PECAM-1 expression, but not claudin-5 or occludin (Fig. 1C). Additionally, co-culture with pericytes improved barrier integrity, as shown by increased TEER (Fig. 1D). All subsequent experiments used this HBMVEC-pericyte co-culture as a 3D BBB model.
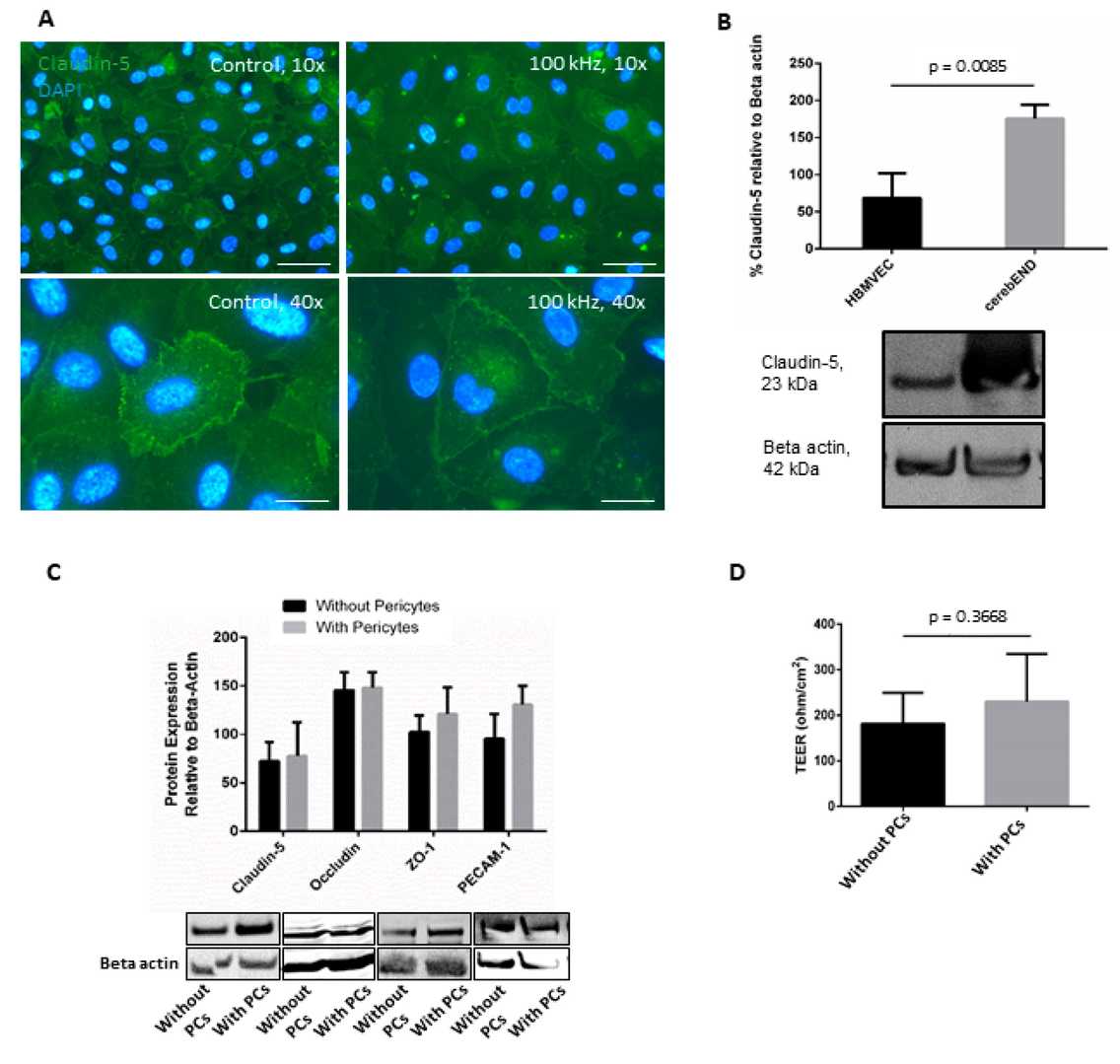 Fig. 1. The addition of pericytes to culture increased HBMVEC barrier properties (Salvador E, Köppl T, et al., 2023).
Fig. 1. The addition of pericytes to culture increased HBMVEC barrier properties (Salvador E, Köppl T, et al., 2023).
BBB-on-a-Chip Containing Pericytes to Model Barrier Disruption
The blood–brain barrier (BBB) restricts substances from entering the CNS, and its dysfunction is linked to various neurological diseases. Traditional models like Transwell have limitations, while microfluidic models face challenges in throughput and material properties. In this study, Ohbuchi et al. developed a BBB-on-a-chip using the OrganoPlate platform, incorporating immortalized TY10 brain endothelial cells, pericytes, and astrocytes. They co-cultured these cells in a microfluidic device to mimic the in vivo BBB structure.
Pericytes play an important role in microvasculature integrity under physiological conditions. To develop a BBB-on-a-chip containing pericytes, pericytes were co-cultured in TY10 microvessels by embedding them in the middle ECM (Fig. 2a). Ten days after TY10 and pericyte co-culture, immunostaining for CD31 (endothelia marker) and platelet-derived growth factor receptor (PDGFR; pericyte marker) was performed. In the co-cultured model, microvessels composed of CD31-positive endothelia were formed, as shown in the TY10 monoculture model in Figure 2b. PDGFR-positive pericytes were localized on the basal side, surrounding the TY10 microvasculature (Fig. 2b). These structures closely resembled those in the in vivo BBB. The BBB-on-a-chip model containing pericytes also exhibited a leak-tight barrier function (Fig. 2c and d). The apparent permeability (Papp) was 6–9 × 10−7 cm/s in the co-culture as compared to 6–7 × 10−6 cm/s in the monoculture model. The TEER was 15 (AM) to 18 (CB) and 9 (CB) to 11 (AM) Ω·cm2, respectively, for the co- and monoculture models (Fig. 2e). These results suggest that the pericytes functionally supported microvascular barrier integrity in our model.
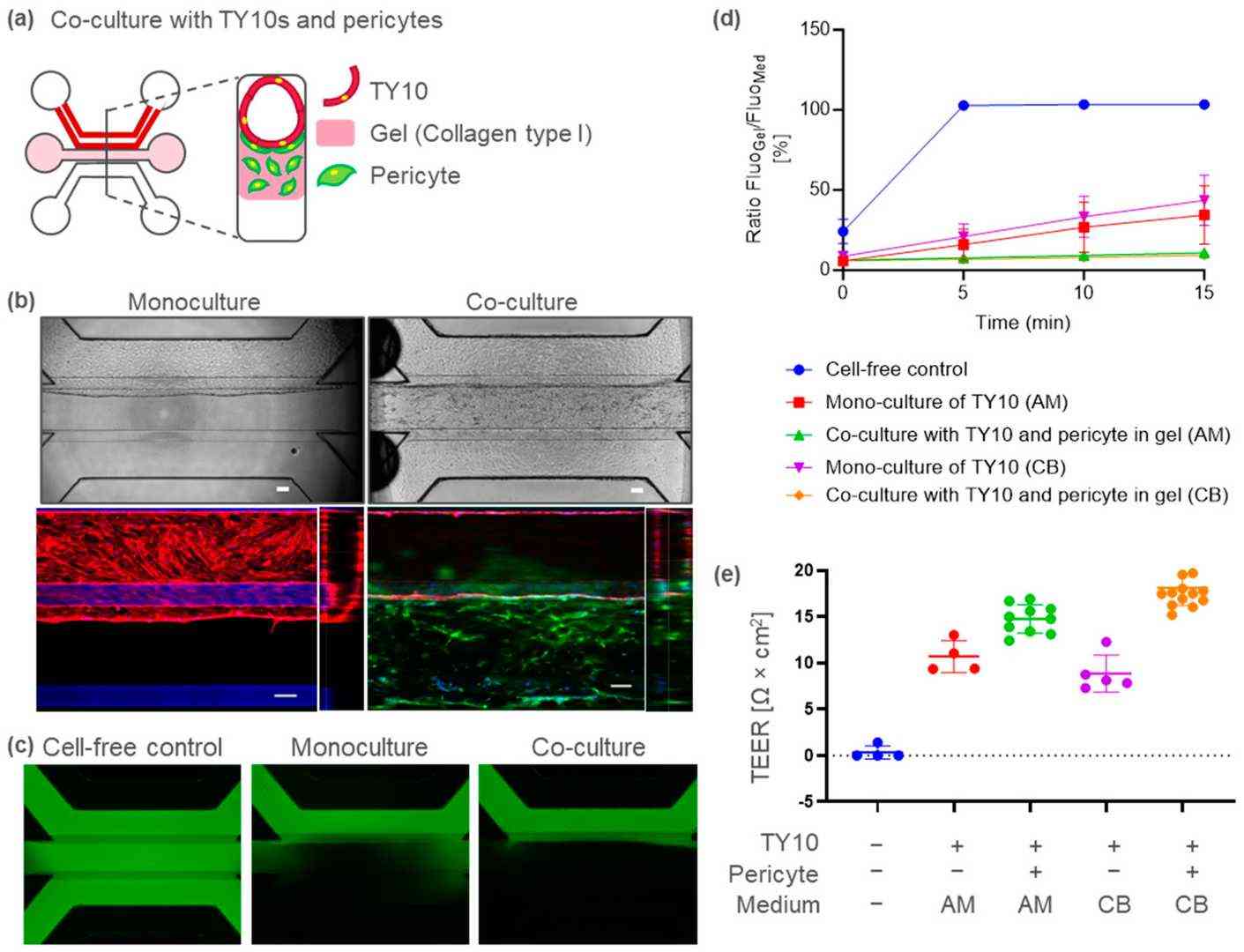 Fig. 2. BBB-on-a-chip model containing TY10 brain endothelia and pericytes (Ohbuchi M, Shibuta M, et al., 2024).
Fig. 2. BBB-on-a-chip model containing TY10 brain endothelia and pericytes (Ohbuchi M, Shibuta M, et al., 2024).
These cells are invaluable for a range of research applications, including vascular biology, angiogenesis studies, tissue engineering, wound healing, and understanding the blood-brain barrier. They also serve as a reliable model for studying pericyte-related pathologies.
Immortalized Human Pericytes offer a consistent and long-lasting model that generates reproducible results, unlike primary pericytes which have a limited lifespan and exhibit donor variability. This consistency is crucial for in-depth and long-term studies.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells