
Immortalized Human Corneal Epithelial Cells
Cat.No.: CSC-I1916Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20°C.
Cultured human corneal epithelial cell lines could provide an efficient model for the study of cellular signaling and molecular pathways regulating normal corneal epithelial cell homeostasis. Previously, cultured human cells lines have been developed for this purpose by transformation with viral oncoproteins including adenovirus E1A, the SV40 large T antigen, and HPV16-E6/E7. The effectiveness of these virally transformed cell lines as potential research models has been hampered by both genetic instability and a lack of normal growth and differentiation properties, preventing their effective use for studying normal epithelial cell biology.
Human cell lines immortalized with hTERT exhibit genetic stability, normal contact inhibition, and maintain the capacity to differentiate. hTERT-immortalized human corneal epithelial cells (HCEs) exhibit a high degree of morphological similarity to primary cultures, expresses a panel of markers associated with corneal epithelial cell function (e.g., ABCG2, cytokeratin 3 and PAX 6 as well as integrins α9 and β1) and respond to epidermal growth factor in proliferation, migration and scratch wound assays. hTERT-immortalized HCEs represents a useful tool for studying many aspects of corneal epithelial cell biology, including regeneration and the response to growth factors, drugs or toxicity, with the added benefit of being able to grow on uncoated substrates in the absence of media supplements typically required for similar cell lines.
L-carnitine Partially Restores Adherens Junction Integrity and Promotes Wound Healing in Human Corneal Epithelial Cells
Disruption of tear film homeostasis and increased osmolarity are key features of dry eye disease (DED), leading to inflammation, epithelial barrier dysfunction, and ocular surface damage. Adherens junctions, primarily composed of cadherins and catenins, are essential for maintaining epithelial integrity and modulating signaling pathways that regulate cell proliferation and migration. This study investigates the effects of hyperosmolarity on adherens junction proteins and wound healing, as well as the therapeutic potential of L-carnitine (LCAR) in mitigating these effects.
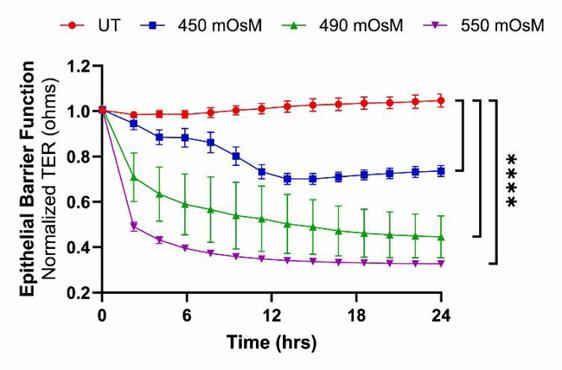
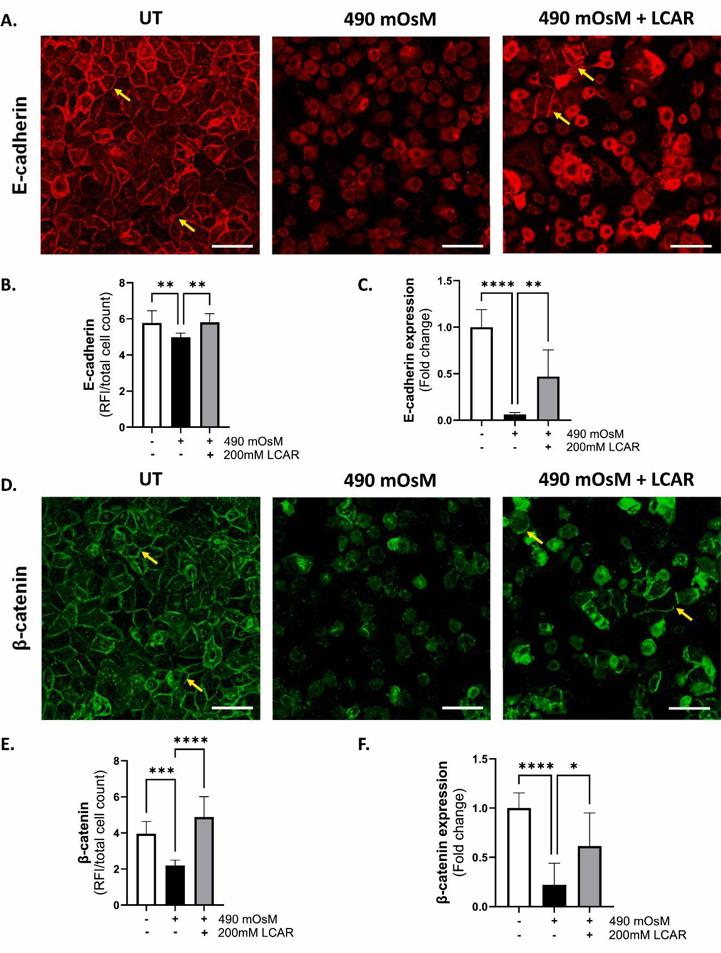
To induce hyperosmolar stress, telomerase-immortalized human corneal epithelial cells (hTCEpi) were treated with 70, 90, and 120 mM NaCl, resulting in final osmolarities of approximately 450, 490, and 550 mOsM, respectively. LCAR supplementation (200 mM) was evaluated as a potential osmoprotective therapy.
Hyperosmolarity caused a dose-dependent reduction in trans-epithelial resistance (TER), with a 30–69 % decline across treatment groups, along with significantly impaired cell migration. Adherens junction proteins (E-cadherin, β-catenin, and p120-catenin) were downregulated, while α-catenin was upregulated. Notably, L-carnitine treatment alleviated these effects, significantly restoring TER and adherens junction protein levels to near-normal. These findings demonstrate that hyperosmolarity impairs corneal epithelial barrier function and delays wound healing by altering adherens junction complex. The results highlight the potential of L-carnitine as a therapeutic agent to restore epithelial barrier integrity and mitigate hyperosmolarity-induced damage in DED.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

