Immortalized Human Adipose Tissue-Derived Mesenchymal Stem Cells-SV40 (Tet-on)
Cat.No.: CSC-I1908Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
CIK-HT004 HT? Lenti-SV40 (tsA58 temperature sensitive mutant) Lentivirus Immortalization Kit
Note: Never can cells be kept at -20 °C.
Immortalized Human Adipose Tissue-Derived Mesenchymal Stem Cells-SV40 (Tet-on) is a modified human adipose tissue-derived mesenchymal stem cell (AD-MSC) cell line that has been stably transfected with the SV40 large T antigen under the control of a tetracycline (Tet)-inducible promoter. The SV40 transgene allows for conditional immortalization of the cells, and the Tet-on system allows for tight regulation of SV40 expression in response to doxycycline (Dox). This enables the cells to be cultured indefinitely, while also maintaining their stem cell properties and can be used to study the effects of controlled cell proliferation on MSC biology.
The cells exhibit typical MSC properties, such as the ability to differentiate into multiple lineages (adirogenic, osteogenic, and chondrogenic) and the expression of MSC surface markers (CD73, CD90, CD105). The Tet-on system allows for tight regulation of SV40 expression, ensuring that the cells can be conditionally immortalized in the presence of Dox and return to a non-immortalized state upon its withdrawal. This feature minimizes the risk of uncontrolled proliferation and genomic instability, making the cell line suitable for long-term studies and applications. This cell line is used in various research areas, including regenerative medicine, tissue engineering, and drug discovery, as it provides a robust and scalable platform for studying MSC biology, mechanisms of senescence, and differentiation.
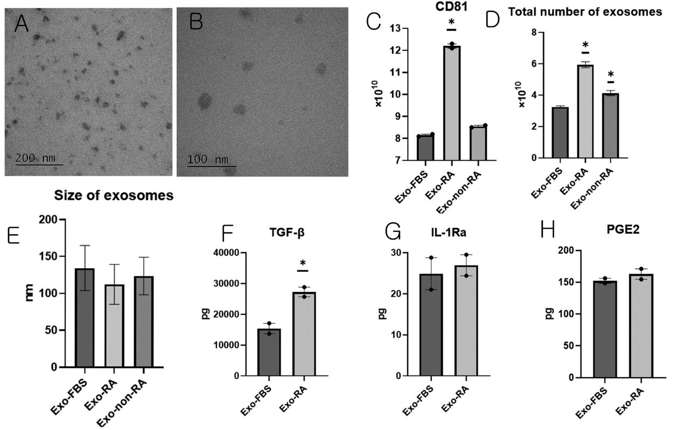
Exosomes Derived from Mesenchymal Stem Cells Primed With Disease-Condition-Serum Enhanced TGF-Β1 Production
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease characterized by synovial inflammation-mediated progressive destruction of the cartilage and bone, resulting in reduced quality of life. Choi et al. primed human telomerase reverse transcriptase-overexpressing immortalized human adipose tissue-derived mesenchymal stem cells (iMSCs) with serum derived from a non-human primate RA model and studied the immunomodulatory ability of exosomes obtained from primed iMSCs.
Exosomes from iMSCs (Exo-FBS), iMSCs with RA serum (Exo-RA), or non-RA serum (Exo-non-RA) were isolated. TEM was used to confirm shape and size (Fig. 1A, B). NTA was used to verify size distribution. CD81 production was the highest in Exo-RA (Fig. 1C). The exosome number and size were the highest and smallest in Exo-RA (Fig. 1D, E). The analysis of exosomes produced from 107 iMSCs showed that the amount of TGF-β1 was 15,376.4 ± 1693.2 pg in Exo-FBS and 27,242.4 ± 1564.8 pg in Exo-RA; the amount of TGF-β1 was significantly higher in Exo-RA than in Exo-FBS (Fig. 1F). The analysis of exosomes produced from 107 iMSCs showed that the amount of IL-1Ra was 24.9 ± 3.9 pg in Exo-FBS and 27.0 ± 2.5 pg in Exo-RA (Fig. 1G). The amount of prostaglandin E2 (PGE2) levels was 152.3 ± 3.9 pg in Exo-FBS and 162.8 ± 8.3 pg in Exo-RA (Fig. 1H).
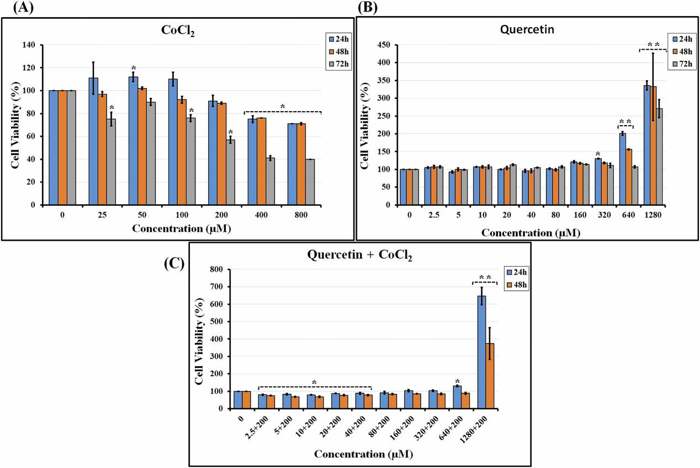
Effect of Quercetin in the Presence of Cocl2 as an Inducing Hypoxia Agent on hTERT-MSC Viability
Studying how small molecules affect stem cell characteristics under normal and low-oxygen conditions is key to optimizing biomedical applications. Aref et al. examined the effects of Quercetin (QC), in combination with hypoxia mimetic CoCl2, on the biological properties of hTERT-MSCs.
MTT assay revealed that 24-h exposure to CoCl2 at ≤ 100 µM increased hTERT-MSC viability, which was significant at 50 µM (Fig. 2A). In contrast, 200–800 µM CoCl2 significantly decreased cell viability at ≥ 400 µM. All concentrations of CoCl2 led to reduced viability of hTERT-MSCs at 48 and 72 h with the greatest decline observed at 72 h. A subsequent MTT assay assessed QC's impact on hTERT-MSC viability. The results showed that QC at 2.5–200 µM did not significantly change cell viability, while 320 µM QC only slightly increased viability at 24 h. However, 640 µM QC significantly increased hTERT-MSC viability at 24 and 48 h (Fig. 2B), while 1280 µM QC increased cell viability at all time points. Notably, the higher the QC concentration, the shorter the treatment time required to increase viability. We also performed MTT assay to determine the combined effects of QC at 2.5–1280 µM and 200 µM CoCl2 on hTERT-MSC viability. QC at ≤ 40 µM significantly decreased viability in a time-dependent manner (Fig. 2C). Cell viability was significantly increased by both 640 and 1280 µM QC with the effects of 1280 µM QC being more pronounced throughout all time points.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells