
RK-13
Cat.No.: CSC-C9125W
Species: Oryctolagus cuniculus (Rabbit)
Source: Kidney
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
RK-13 is an epithelial-like adherent cell line which originated from the renal cortex of New Zealand white rabbits. Cells have a regular morphology, growing in a cobblestone-like monolayer. RK-13 can be stably proliferated in MEM or DMEM medium with the addition of 10% fetal bovine serum. RK-13 cells are highly susceptible to a broad spectrum of viruses, especially Herpesviridae and Poxviridae pathogens, including rabbitpox virus and pseudorabies virus. Researchers widely use the RK-13 cell line for conducting viral cultures and developing vaccines alongside testing antiviral medications. The RK-13 cell line also has high transfection efficiency, and is often used for viral vector construction and gene expression studies. Despite its restricted adaptability to human viruses RK-13 remains an essential research tool for veterinary and comparative medicine studies.
Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using Rabbit Kidney (RK-13) Cells
Equine herpesvirus type 8 (EHV-8) is predominantly isolated from donkeys, but its biological properties and pathogenic potential remain underexplored. Ji’s team investigated the biological characteristics and pathogenicity of the EHV-8 LCDC01 isolate by examining its effects in rabbit kidney (RK-13) cells and BALB/c mice.
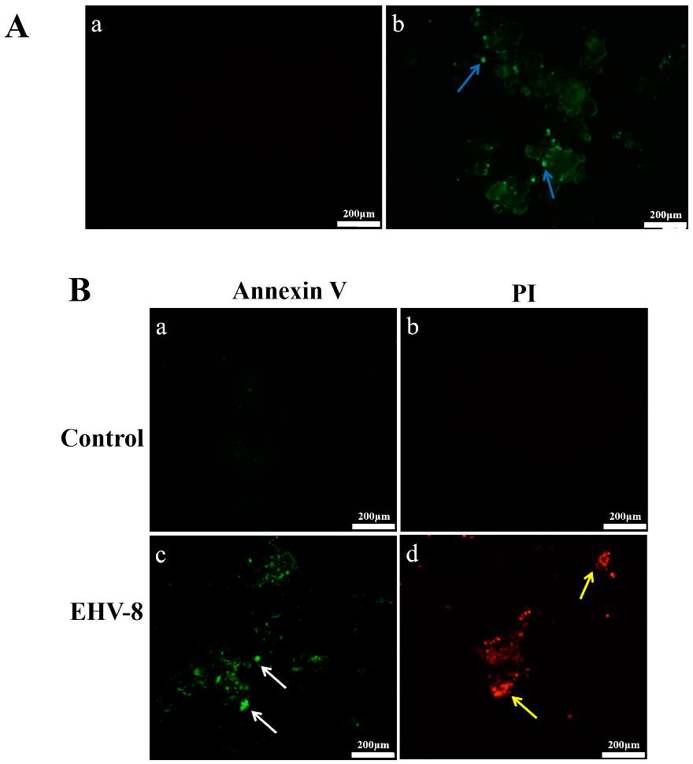
Figure 1 shows the infection of EHV-8 and the effect of EHV-8 on RK-13 cells. Figure 1A shows the results of immunofluorescence. EHV-8-specific fluorescence was abundant in the infected cells (Fig. 1(Ab)), and the control group had no signal (Fig. 1(Aa)). These results showed that EHV-8 could efficiently infect and replicate in RK-13 cells. Figure 1B shows apoptosis after infection. The number of green fluorescence (white arrows) positive cells in the early apoptosis group in the infected group (Fig. 1(Bc)) was significantly increased compared to the control group (Fig. 1(Ba)). The number of red fluorescence (yellow arrows) positive cells in the late apoptosis/necrosis group in the infected group (Fig. 1 (Bd)) was significantly increased compared to the control group (Fig. 1(Bb)). These data showed that EHV-8 infection could effectively induce apoptosis of RK-13 cells.
Inactivation of Equine Arteritis Virus by Indolicidin Peptide
Equine viral arteritis is an economically important contagious disease caused by equine arteritis virus (EAV). Metz et al. evaluated the antiviral activity of indolicidin and K-indolicidin peptides against EAV.
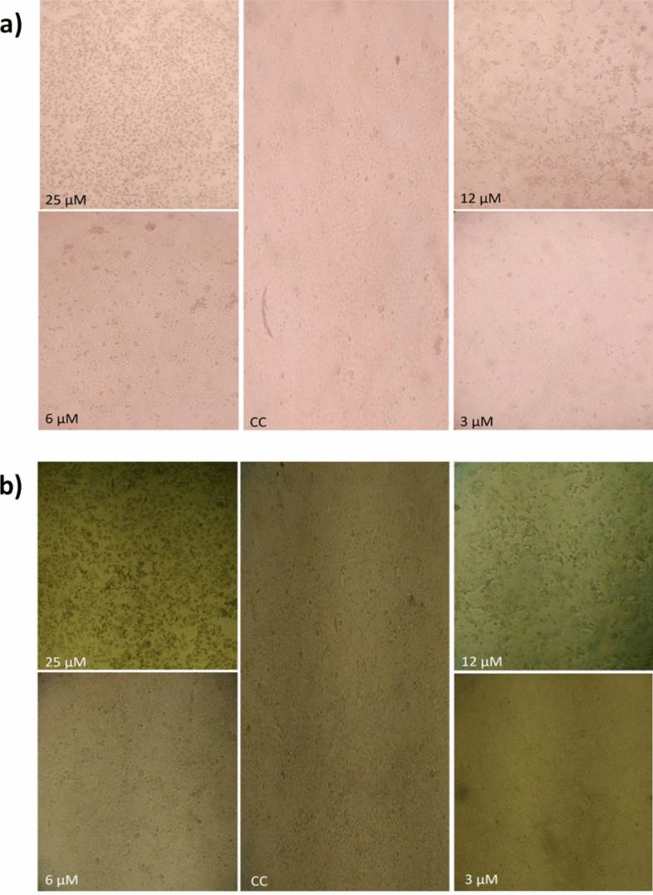
Cytotoxicity of Indolicidin and K-indolicidin was first screened on EAV-susceptible RK-13 and Vero CCL-81 cells. Within 24 h, 50 µM detached all cells; 25 and 12 µM were severely toxic, whereas 6 µM only altered morphology without loss of viability (confirmed by Trypan Blue; Fig. 2a). No effect was seen at 3 µM. Therefore, 5 µM (sub-toxic) was selected for antiviral tests. At this concentration, Indolicidin completely prevented EAV CPE and plaque formation in RK-13 cells at MOI = 1, indicating full inhibition of replication. K-indolicidin lacked activity, presumably because the extra N-terminal lysine changes net charge and target interaction. Antiviral efficacy of Indolicidin remained consistent in Vero cells but declined as viral inoculum increased (Fig. 3).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
