
Immortalized Mouse Bone Marrow Macrophages-SV40
Cat.No.: CSC-I2161Z
Species: mouse
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Note: Never can cells be kept at -20°C.
Immortalized Mouse Bone Marrow Macrophages-SV40 is a cell line that originates from the bone marrow of mice. The bone marrow is a primary hematopoietic organ found within the bones of the body. It is responsible for producing blood cells, including red blood cells, white blood cells, and platelets. Macrophages in the bone marrow are a crucial component of the immune system and play a key role in pathogen clearance, immune response modulation, and tissue homeostasis. The large T-antigen gene from SV40 virus transformed this cell line to enable endless cell proliferation.
This cell line is suited for constructing models of autoimmune diseases, infectious diseases, and tumor microenvironments, facilitating studies into disease mechanisms and potential therapeutic strategies. It can also be used to study the role of macrophages in the immune system, including antigen presentation, T cell activation, and inflammatory responses. In addition, this cell line can be used to study the regulatory mechanisms of signaling pathways in macrophages, such as NF-κB, MAPK, and JAK/STAT, and their roles in various diseases.
Structural Mechanisms of Calmodulin Activation of Shigella Effector Ospc3 to ADP-Riboxanate Caspase-4/11 and Block Pyroptosis
Pyroptosis is a programmed cell death essential for antibacterial immunity that is triggered by the caspase-4/11-GSDMD axis. OspC3 effector from Shigella flexneri antagonizes pyroptosis through ADP-riboxanation of caspase-4/11. However, the structural basis by which calmodulin activates OspC3 to catalyze this modification and inhibit pyroptosis remains unclear.
Here, Huo et al. identified Ca2+-free calmodulin (CaM) that binds and activates the ADP-riboxanase activity of OspC3. The crystal structures of OspC3–CaM and OspC3–caspase-4 complexes, as well as the CaM–OspC3–caspase-4 ternary complex, reveal the unique binding of CaM to the N-terminal domain of OspC3, specific recognition of caspase-4 by the ankyrin repeat domain of OspC3, and NAD+ binding-triggered reorganization of the catalytic pocket. Within this pocket, D231 and D177 of OspC3 activate the substrate arginine for ADP-ribosylation and downstream deamination. Shigella infection was used to validate the functional significance of our structural discoveries. In S. flexneri, ΔospC3 triggered GSDMD cleavage and pyroptosis in human A431 cells and mouse iBMMs, which was mitigated by re-expression of WT OspC3 (Fig. 1a). In contrast, mutants defective in caspase-4/11 recognition (L414D/V449D), CaM binding (V59D/F62D/L63D), or NAD+ loading and arginine activation (F188A/F211A, E326A, D231A) failed to block the caspase-4/11-GSDMD axis (Fig. 1a). The ΔospC3 strain exhibited a replication defect in mouse livers and spleens, which permitted mouse survival (Fig. 1b and c). These OspC3 mutants, in contrast to WT OspC3, failed to rescue this defect of ΔospC3 in mouse tissues and similarly permitted mouse survival like ΔospC3-infected mice (Fig. 1b and c). These results validate the functional relevance of CaM binding and caspase-4/11 recognition for OspC3 activation.
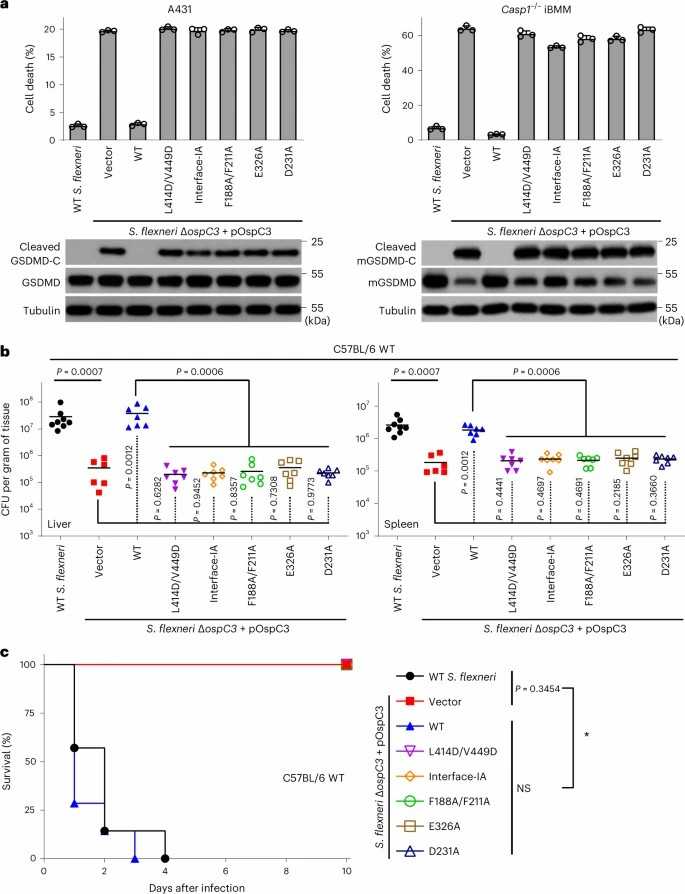 Fig. 1. Functional analyses of structural mechanisms for CaM activation of OspC3 to target and ADP-riboxanate caspase-4/11 (Huo Y J, Zeng H, et al., 2023).
Fig. 1. Functional analyses of structural mechanisms for CaM activation of OspC3 to target and ADP-riboxanate caspase-4/11 (Huo Y J, Zeng H, et al., 2023).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
