- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
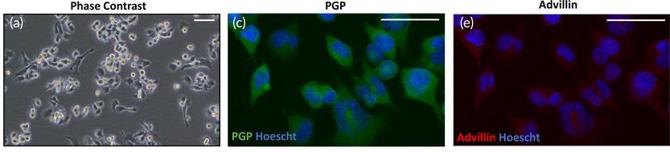
F11 is a somatic hybrid cell line, the product of the fusion between the mouse neuroblastoma line N18TG‑2 and embryonic rat dorsal‑root‑ganglion (DRG) neurons. This dual‑species origin endows the cells with a neuronal phenotype that closely resembles peripheral sensory neurons while retaining the robust proliferative capacity of a tumor‑derived line. When grown in standard DMEM with 10 % fetal bovine serum, F11 cells form a monolayer of adherent cells. Exposure to low‑serum medium with the cyclic‑AMP analogues (forskolin) and nerve‑growth factor causes F11 cells to sprout many long neurites and express neuronal markers like β‑III‑tubulin, NF‑160 and NeuN.
Functionally, they have been found to express the characteristic DRG panel of receptors such as μ‑ and δ‑opioid receptors, bradykinin, prostaglandin, and voltage‑gated Ca²⁺ channels, as well as KCNQ/M (Kv7) potassium channels and TRPV1‑related signaling cascades. Electrophysiologically, the application of opioid receptor agonists causes an increase in voltage‑dependent K⁺ currents that is blocked by pertussis toxin, consistent with G‑protein coupling. The above properties make F11 a popular in vitro model system for studies on pain‑modulation, opioid pharmacology, ion‑channel screening, and neurotoxicology (e.g., chemotherapy‑induced peripheral neuropathy). F11 cells have become a popular platform for peripheral neuronal signaling studies due to their ability to differentiate and express sensory-neuron specific signaling pathways easily in culture.
Ascl1-Mediated Enhancement of Gabaergic Neuronal Function in Differentiated F11 Cells under High Glucose Conditions
Gamma-aminobutyric acid (GABA)ergic neurons play a key role in pain modulation within the dorsal root ganglion (DRG), making them critical targets for therapeutic studies. Go et al. utilized F11 cells as an in vitro model to examine GABAergic function under high-glucose conditions mimicking diabetic neuropathy.
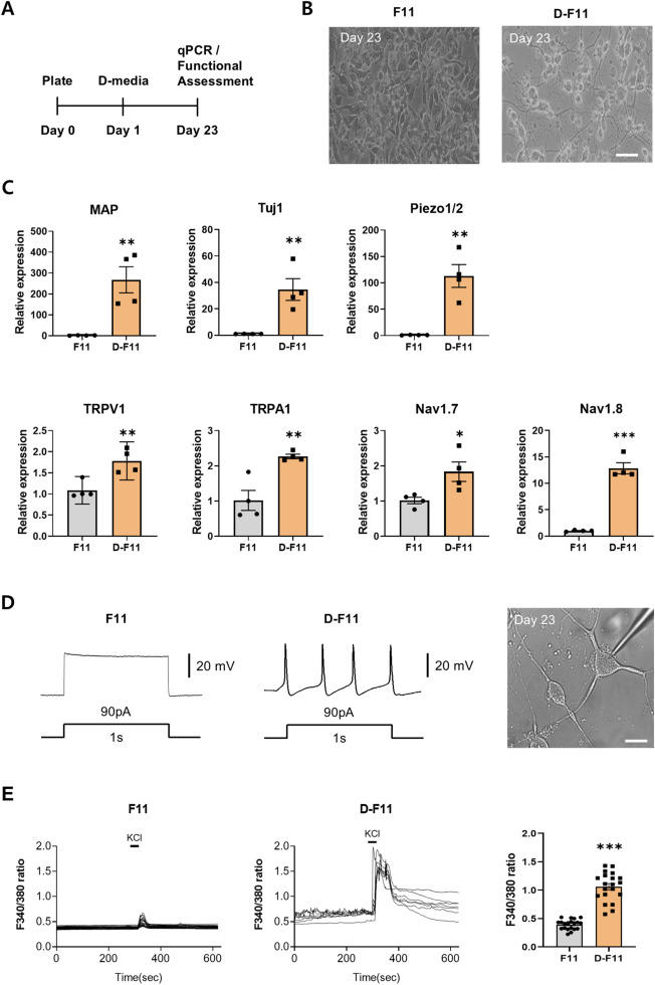
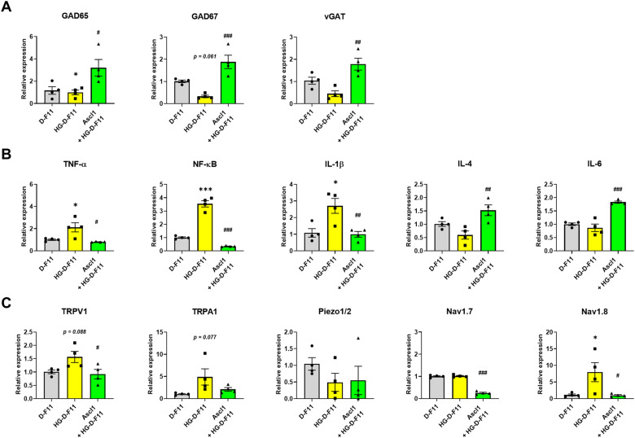
After 23 days in differentiation media, F11 cells showed a neuron-like shape, transitioning from undifferentiated (F11) to differentiated (D-F11) state (Fig. 1A and B). This was confirmed by increased expression of neuronal markers MAP and Tuj1, and sensory neuronal genes TRPV1, TRPA1, Piezo1/2, Nav1.7, and Nav1.8 (Fig. 1C). Whole-cell patch-clamp recordings showed D-F11 cells had spontaneous action potentials unlike inactive F11 cells (Fig. 1D). Calcium imaging revealed D-F11 cells had a significant increase in the F340/380 ratio upon 50 mM KCl stimulation, unlike F11 cells with minimal calcium influx (Fig. 1E). These results show D-F11 cells have mature sensory neuron characteristics. To study Ascl1's role in GABAergic differentiation, Ascl1 was overexpressed in D-F11 cells using lentiviral transduction. For high-glucose (HG) conditions, cells were incubated in 45 mM glucose for 24 hours. In HG-D-F11 cells, GABAergic gene expression (GAD65, GAD67, vGAT) was reduced but restored by Ascl1 treatment (Fig. 2A). Pro-inflammatory cytokines (TNF-α, NF-κB, IL-1β, IL-6) were elevated in HG conditions and suppressed by Ascl1. Anti-inflammatory cytokines (IL-4, IL-10) were unchanged in HG-D-F11 cells but upregulated by Ascl1 (Fig. 2B). Pain-related ion channels (TRPV1, TRPA1, Nav1.8) were increased in HG-D-F11 cells, but Ascl1 overexpression reduced their expression, with significant reductions in TRPV1 and Nav1.8 (Fig. 2C). These findings suggest Ascl1 promotes GABAergic gene expression, suppresses inflammation, and modulates pain channel expression under HG conditions.
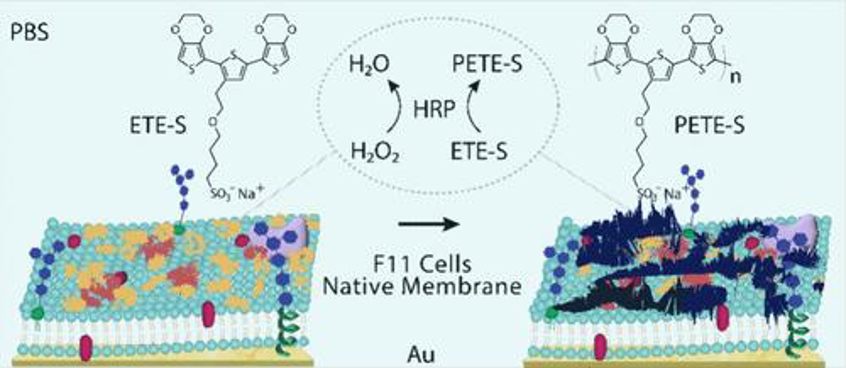
Enzymatically Polymerized Organic Conductors on Native Lipid Membranes
Conductive polymers, with their ability to conduct ions and electrons and flexible mechanical properties, are ideal for bioelectronic applications. Priyadarshini et al. aim to explore the in situ enzymatic polymerization of water-soluble π-conjugated monomers on native lipid bilayers from the F11 cell line, mimicking mammalian neural membranes, to develop minimally invasive neural electrodes for diagnosing and treating neurological disorders.
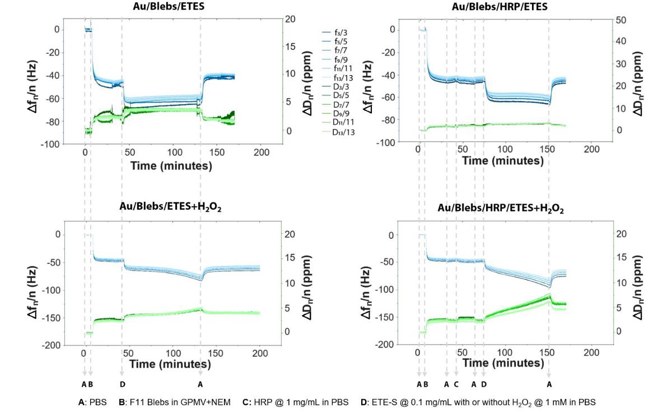
Real-time QCM-D was used to study the adsorption of F11 blebs, HRP, and ETE-S with H2O2 on the Au/Ti quartz sensor. ETE-S is polymerized by HRP to form PETE-S (Fig. 3). HRP has broad substrate selectivity and can polymerize ETE-S using H2O2 as the oxidant. After each adsorption step, a PBS buffer rinse was performed and EIS measurements were recorded. Control measurements were also taken without HRP and H2O2. The entire QCM-D process, including F11 bleb bilayer formation, HRP adsorption, ETE-S adsorption, and polymerization, is shown in Figure 4. QCM-D frequency shifts indicate changes in the film's hydrated mass, while dissipation shifts reflect changes in its viscoelastic properties. A frequency drop signifies increased adsorbed mass, and a dissipation rise indicates increased film softness. Lower overtones represent changes in the top-most surface of the adsorbed material.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells