OC 316
Cat.No.: CSC-6307W
Species: Homo sapiens (Human)
Source: Ascites Metastasis
Morphology: continuous culture, grown as monolayer, morphology epithelial-like
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Tissue: ovary;
Tumor: adenocarcinoma, low differentiated;
G3 FIGO stage IV (pleural metastasis);
Derived from: ascitic fluid
OC316 cells were originally obtained from the ascites of a 60-year-old woman diagnosed with ovarian adenocarcinoma with pleural metastasis. The ovary is a female reproductive organ responsible for the production of ova and secretion of female hormones. Ovarian adenocarcinoma is a common ovarian epithelial tumor that arises from the surface epithelium or glands of the ovary. The disease is characterized by high heterogeneity and invasiveness. It often results in intraperitoneal metastasis and the formation of ascites.
OC316 cells can form tumors when injected either subcutaneously or intraperitoneally into nude mice. The tumors that arise in these mice are highly invasive and can infiltrate the peritoneum. Since the OC316 cell line is resistant to a variety of chemotherapeutic drugs, it can be used as a model for investigating the molecular mechanisms of ovarian cancer resistance and for screening new anti-resistance drugs. The high tumorigenicity of OC316 cells in nude mice also makes this cell line suitable for constructing animal models of ovarian cancer. These models can be used to study the growth and development of ovarian cancer tumors and test the efficacy of potential treatments.
PDK Silencing Modulates Glycolysis and In Vitro Growth of Ovarian Cancer Cells
Glycolysis may promote tumor growth by modulating the stromal microenvironment and enhancing angiogenesis. PDK1, a key regulator of glycolysis, has been studied mainly in vitro. Here, Venturoil et al. investigated the effects of PDK1 silencing on tumor growth, angiogenesis, and metabolism. they silenced PDK1 in OC316 and OVCAR-3 ovarian cancer cells using lentiviral shRNA vectors (Fig. 1A). The efficiency of the silencing in terms of PDK1 protein expression was confirmed by western blot (Fig. 1B). The silencing efficiency was maintained for at least 21 days in vitro (Fig. 1C). As expected, PDK1 silencing resulted in decreased lactate production in the supernatants of the PDK1-silenced cells (Fig. 2A). This was accompanied by a slight increase in OCR and a decrease in ECAR in the Seahorse analysis (Fig. 2B). These changes in energy metabolism were coupled to decreased cell proliferation, as observed in the SRB assay after 72 and 96 h (Fig. 3A) and confirmed by PKH26 staining (Fig. 3B). In conclusion, stable silencing of PDK1 in ovarian cancer cells is associated with decreased glycolysis and proliferation in vitro.
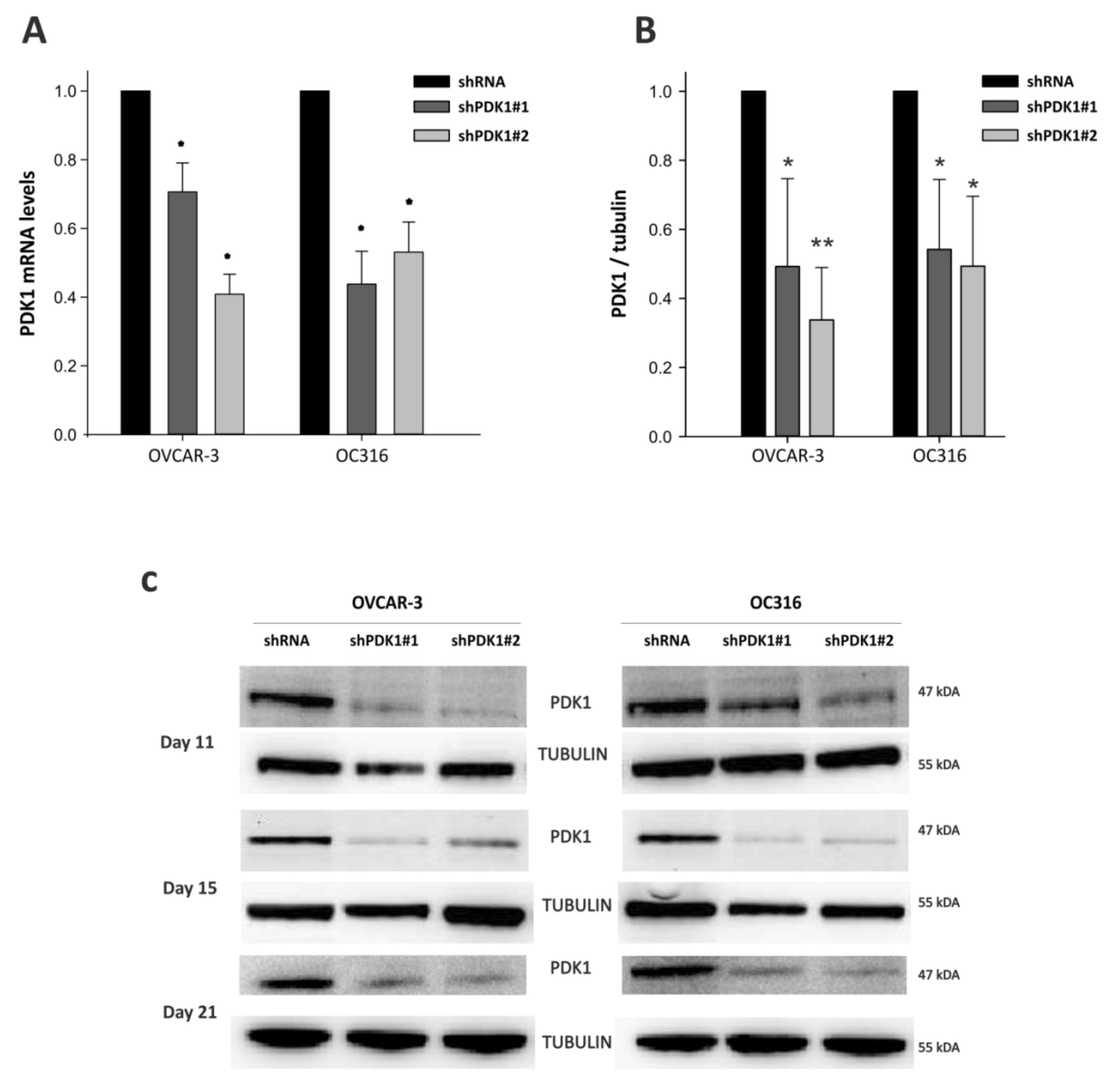 Fig. 1. Decreased expression of Pyruvate dehydrogenase kinase 1 (PDK1) at mRNA and protein levels. OVCAR-3 and OC316 cell lines were transduced by lentiviral vectors encoding PDK1-targeting shRNA (shPDK1#1 and shPDK1#2) (Venturoli C, Piga I, et al., 2021).
Fig. 1. Decreased expression of Pyruvate dehydrogenase kinase 1 (PDK1) at mRNA and protein levels. OVCAR-3 and OC316 cell lines were transduced by lentiviral vectors encoding PDK1-targeting shRNA (shPDK1#1 and shPDK1#2) (Venturoli C, Piga I, et al., 2021).
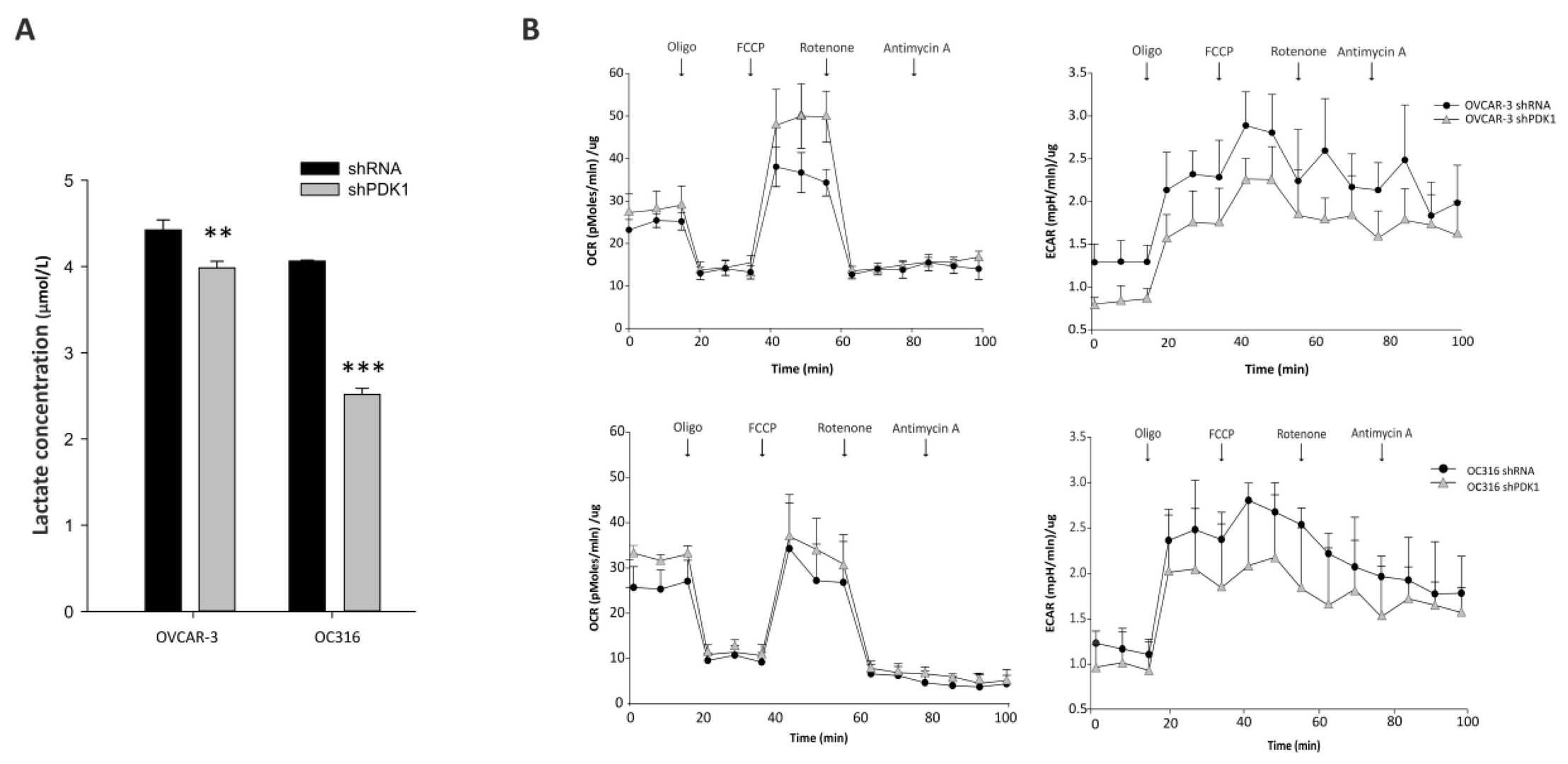 Fig. 2. Effects of PDK1 silencing on glycolysis and cell metabolism (Venturoli C, Piga I, et al., 2021).
Fig. 2. Effects of PDK1 silencing on glycolysis and cell metabolism (Venturoli C, Piga I, et al., 2021).
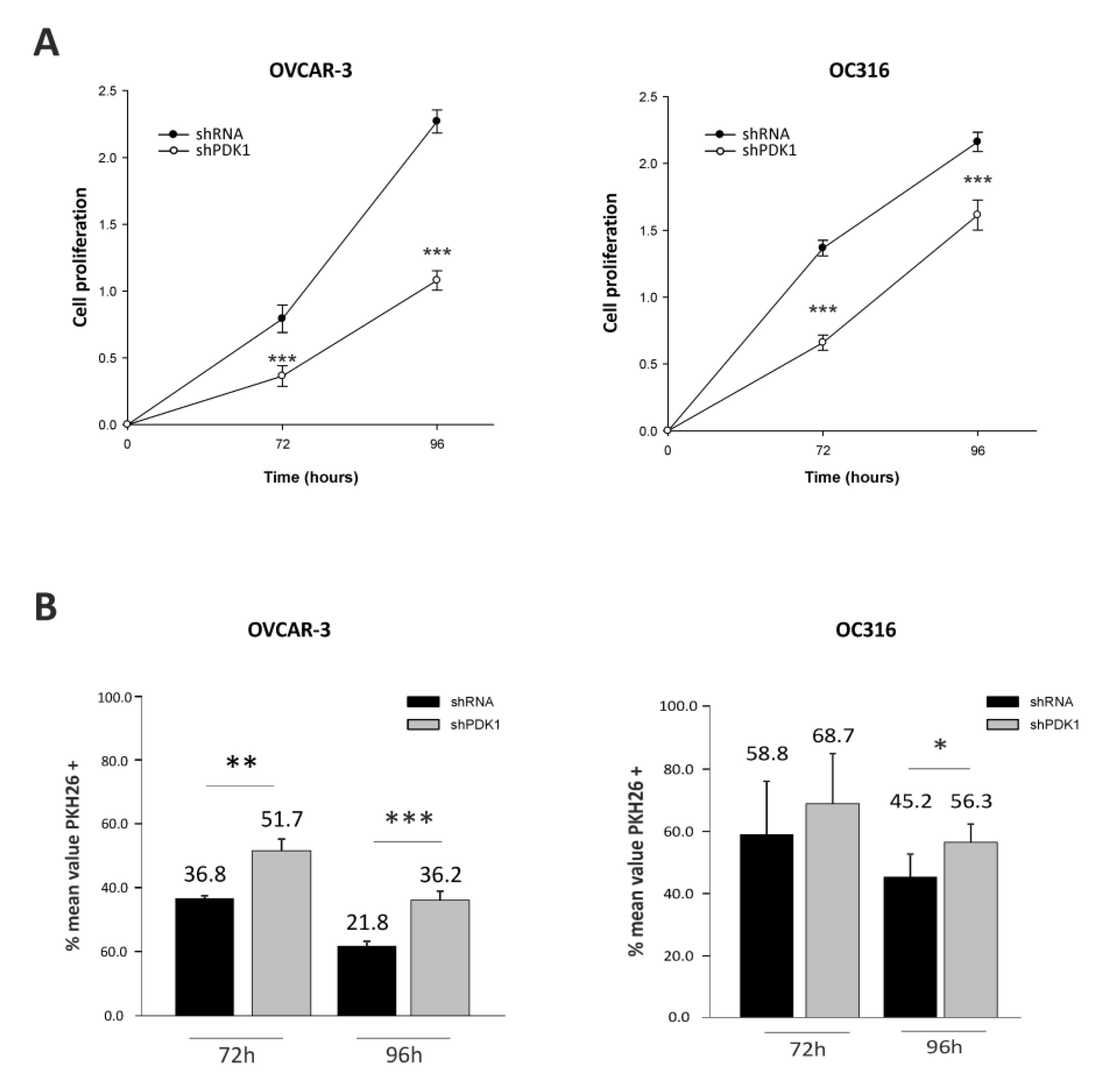 Fig. 3. Effects of PDK1 silencing on cell proliferation (Venturoli C, Piga I, et al., 2021).
Fig. 3. Effects of PDK1 silencing on cell proliferation (Venturoli C, Piga I, et al., 2021).
ARN-3261 Inhibits Cell Growth and Increases Sensitivity to Carboplatin in Ovarian Cancer Cells
Previous studies have shown that treatment with ARN-3261, a small molecule inhibitor of the enzyme salt-induced kinase 2, can improve the response to paclitaxel in human ovarian cancer cells grown in culture and in immunocompromised mice. Here, Fan's team have asked whether inhibition of SIK2 with ARN-3261 enhances sensitivity to carboplatin in ovarian cancer cell lines and xenograft models.
To test if ARN-3261 inhibits ovarian cancer cell growth, its effect was measured in eight ovarian cancer cell lines using short-term proliferation assays. The IC50 of ARN-3261 was calculated for each cell line (Fig. 4A), showing significant dose-dependent inhibition with IC50 values ranging from 0.8 to 3.5 µM. Carboplatin's IC50 values for these cell lines ranged from 1.2 to 34.2 µM (Fig. 4B). When ARN-3261 was added to carboplatin, the carboplatin dose-response curve shifted left in seven of eight cell lines (Fig. 4C), indicating ARN-3261 sensitizes cells to carboplatin. Multi-point drug combination studies in four responsive cell lines (IGROV1, OC316, OVCAR8, and SKOv3) showed combination index (CI) values less than one (Fig. 4D), supporting that SIK2 inhibition enhances carboplatin sensitivity. To exclude off-target effects, SIK2 was knocked down with CRISPR/Cas9 in OVCAR8 and SKOv3 cells, which similarly sensitized cells to carboplatin (Fig. 4E). Clonogenic assays in five cell lines showed ARN-3261 significantly enhanced carboplatin-induced loss of clonogenic survival (Fig. 4F). Overall, these results suggest that SIK2 inhibition potentiates carboplatin's effects, and ARN-3261 synergizes with carboplatin to kill ovarian cancer cells.
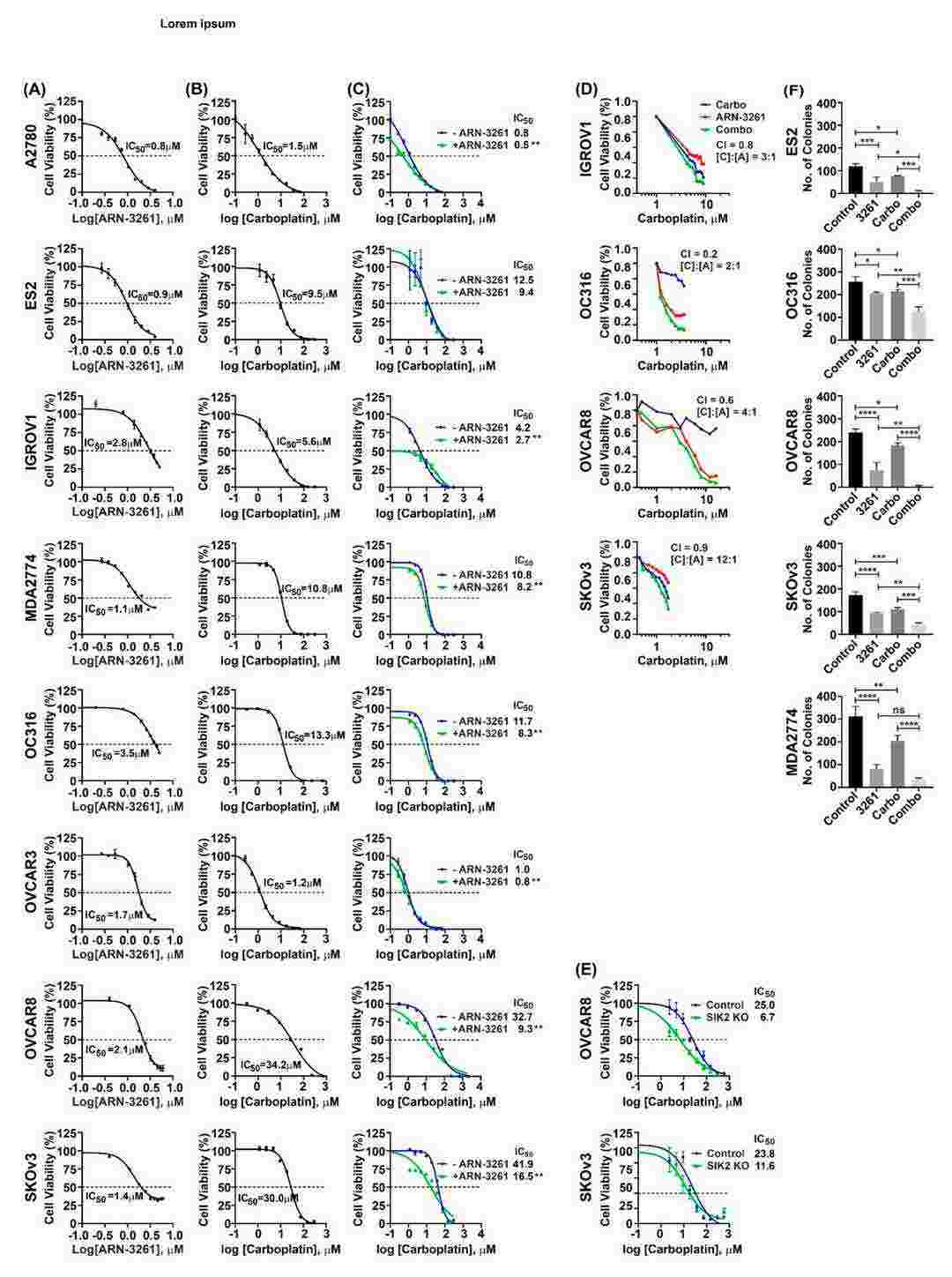 Fig. 4. ARN-3261 synergistically enhances carboplatin-induced inhibition of ovarian cancer cell short term and clonogenic growth in cell culture (Fan D, Yang H, et al., 2021).
Fig. 4. ARN-3261 synergistically enhances carboplatin-induced inhibition of ovarian cancer cell short term and clonogenic growth in cell culture (Fan D, Yang H, et al., 2021).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells