EFO-27
Cat.No.: CSC-C0318
Species: Homo sapiens (Human)
Source: Omentum Metastasis
Morphology: adherent, epithelial-like cells growing as monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin +, cytokeratin-7 +, cytokeratin-8 +, cytokeratin-17 -, cytokeratin-18 +, cytokeratin-19 +, desmin -, endothel -, EpCAM +, GFAP -, neurofilament -,
The EFO-27 cell line, established in 1993 from malignant ascites of a 56-year-old patient with poorly differentiated ovary adenocarcinoma, represents a valuable model for studying high-grade serous ovarian carcinoma (HGSC), the most common and aggressive subtype. Derived from a chemotherapy-naive patient, EFO-27 exhibits typical epithelial morphology, and harbors a homozygous TP53 mutation (R248Q), a near-universal feature of HGSC. Key characteristics include expression of epithelial markers (cytokeratins, EpCAM), lack of functional p53, and sensitivity to platinum-based drugs (cisplatin, carboplatin) in vitro, although it can develop resistance. Current studies leverage EFO-27 mainly to explore: 1) Mechanisms of therapeutic resistance: Modeling intrinsic and acquired resistance to platinum chemotherapy and PARP inhibitors (PARPis), crucial for understanding treatment failure in HGSC. 2) Novel therapeutic strategies: Evaluating the efficacy and mechanism of new drugs (targeted therapies, antibody-drug conjugates), immunotherapy combinations (e.g., checkpoint inhibitors), and strategies to overcome resistance or resensitize tumors. 3) Tumor microenvironment interactions: Studying interactions with stromal cells, immune evasion mechanisms, and the impact of the ascites environment using co-culture models or xenografts. 4) Biomarker discovery: Identifying molecular signatures associated with drug response or resistance pathways.
Onvansertib and Navitoclax Combination as a New Therapeutic Option for Mucinous Ovarian Carcinoma
Mucinous epithelial ovarian cancer (mEOC) is a rare subtype of epithelial ovarian cancer, characterized by poor responses to standard platinum-based chemotherapy. Polo-like kinase 1 (PLK1) is a key regulator of mitosis and cell cycle progression and its inhibition has been recently identified as a target in mEOC. In this study, we aimed to identify further therapeutic targets in mEOC using a CRISPR/Cas9 library targeting 3015 genes, with and without treatment with onvansertib, a PLK1 inhibitor. We identified twelve genes associated with cell survival (ZC2HC1C, RPA2, KIN17, TUBG1, SMC2, CDC26, CDC42, HOXA9, TAF10, SENP1, MRPS31, and COPS2) and three genes (JUND, CARD9, and BCL2L2) in synthetic lethality with onvansertib treatment. We validated that SENP1 downregulation is important for the growth of mEOC cells through esiRNA interference and the use of a pharmacological inhibitor Momordin Ic. The downregulation of CARD9 and BCL2L2 combined with subtoxic doses of onvansertib interfered with mEOC cell growth. Interestingly, the combination of navitoclax, an inhibitor of BcL2 family members including BCL2L2, was synergistic in all four of the mEOC cell lines tested and substantially induced cell death through apoptosis. These data support the use of a combination of navitoclax and onvansertib as a new therapeutic strategy for mEOC.
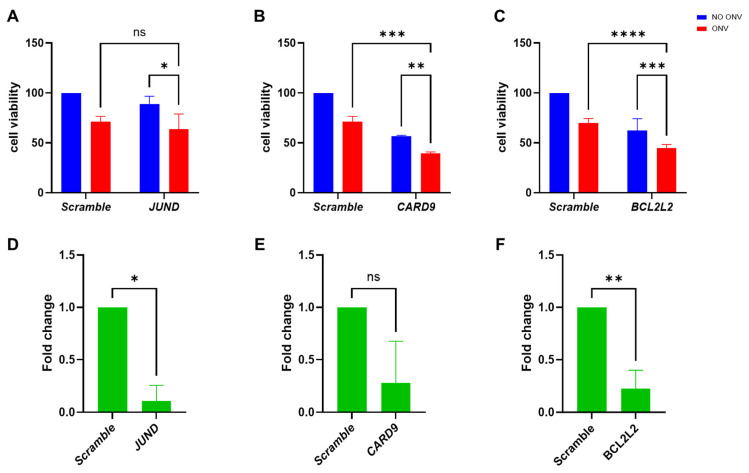 Fig. 1. Cellular viability of EFO27 cells transfected with the esiRNAs JUND, CARD9, and BCL2L2 with or without onvansertib treatment (Petrella, Serena, et al. 2025).
Fig. 1. Cellular viability of EFO27 cells transfected with the esiRNAs JUND, CARD9, and BCL2L2 with or without onvansertib treatment (Petrella, Serena, et al. 2025).
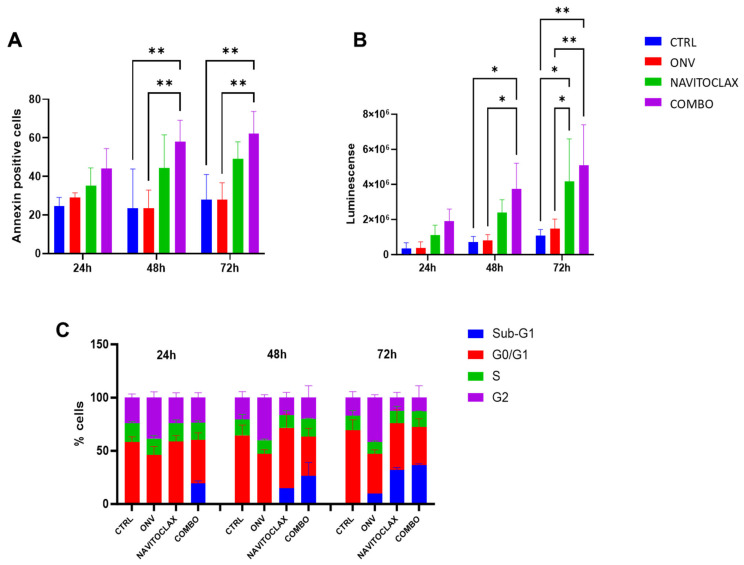 Fig. 2. Combination of navitoclax and onvansertib on EFO27 cells (Petrella, Serena, et al. 2025).
Fig. 2. Combination of navitoclax and onvansertib on EFO27 cells (Petrella, Serena, et al. 2025).
A Comprehensive Analysis of Modified Nucleosides in Ovarian Cancer Cell Lines
Ovarian carcinoma is a gynecological cancer with poor long-term survival rates when detected at advanced disease stages. Early symptoms are nonspecific, and currently, there are no adequate strategies to identify this disease at an early stage when much higher survival rates can be expected.
Ovarian carcinoma is a heterogeneous disease, with various histotypes originating from different cells and tissues, and is characterized by distinct somatic mutations, progression profiles, and treatment responses. This study presents a targeted metabolomics approach, characterizing seven different ovarian (cancer-) cell lines according to their extracellular, intracellular, and RNA-derived modified nucleoside profiles. Moreover, these data were correlated with transcriptomics data to elucidate the underlying mechanisms. Modified nucleosides are excreted in higher amounts in cancer cell lines due to their altered DNA/RNA metabolism. The study shows that seven different ovarian cancer cell lines, representing different molecular subtypes, can be discriminated according to their specific nucleoside pattern. Modified nucleosides are suggested as strong biomarker candidates for ovarian cancer with the potential for subtype-specific discrimination. Extracellular modified nucleosides have the highest potential in the distinguishing of cell lines between control cell lines and themselves, and represent the closest to a desirable, non-invasive biomarker, since they accumulate in blood and urine.
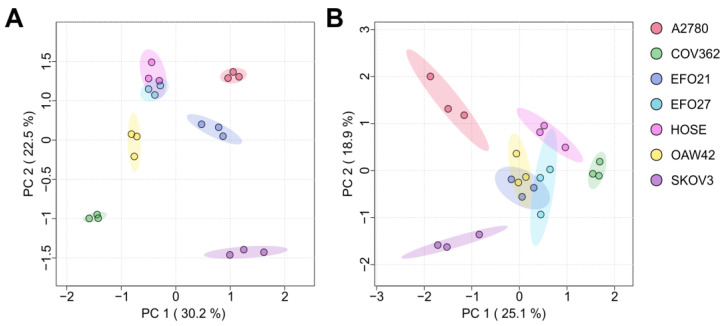 Fig. 3. The PCA (principal component analysis) plots of the exo- (A) and endo-nucleosides (B) in both the cell culture medium and cells (Mohl, D. A., et al. 2025).
Fig. 3. The PCA (principal component analysis) plots of the exo- (A) and endo-nucleosides (B) in both the cell culture medium and cells (Mohl, D. A., et al. 2025).
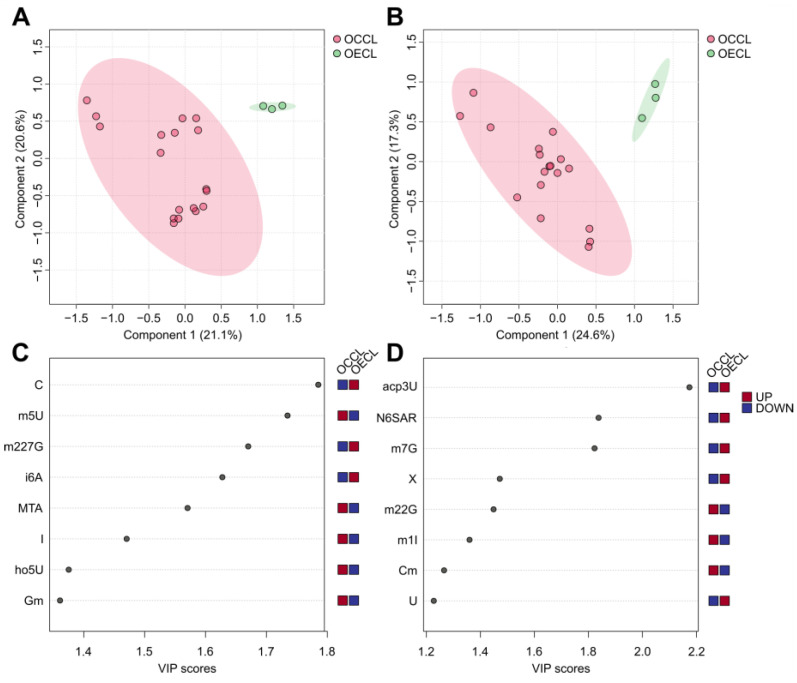 Fig. 4. The exo- (A) and endo-nucleoside (B) analysis of the ovarian epithelial cell line (OECL) and ovarian cancer cell line (OCCL) (Mohl, D. A., et al. 2025).
Fig. 4. The exo- (A) and endo-nucleoside (B) analysis of the ovarian epithelial cell line (OECL) and ovarian cancer cell line (OCCL) (Mohl, D. A., et al. 2025).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells