
Immortalized Mouse Dopaminergic Neuronal Cells (MN9D)
Cat.No.: CSC-I2295Z
Species: mouse
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
MN9D is an immortalized murine dopaminergic neuronal cell line created by somatic cell fusion of dopamine neurons from mouse embryonic ventral midbrain with neuroblastoma cells (N18TG2). Morphologically, they are immature neurons, but when cultured in special conditions can be induced to extend neurites and differentiate (e.g. by treatment with all-trans retinoic acid (tRA)). Although they appear as immature neurons morphologically these cells show dopaminergic differentiation features like tyrosine hydroxylase (TH) expression and dopamine production capacity under appropriate conditions.
MN9D cells express dopamine and related enzymes which makes them useful for creating dopaminergic neuron injury models including neurodegeneration models for diseases like Parkinson's. MN9D cells are also commonly used to study the neurotoxic effects of various neurotoxic compounds (such as 6-hydroxydopamine (6-OHDA), MPP+, etc.) on dopaminergic neurons, as well as the protective effects of antioxidants and other neuroprotectants.
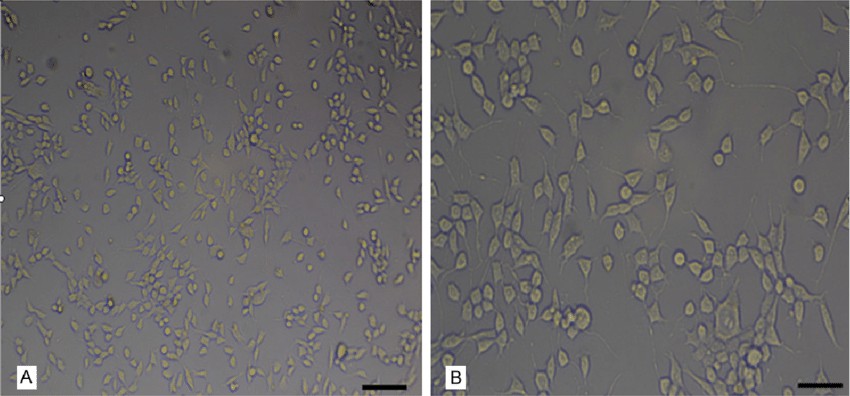 Fig. 1. Morphological characteristics of MN9D cells (Tian P X, Shi W B, et al., 2015).
Fig. 1. Morphological characteristics of MN9D cells (Tian P X, Shi W B, et al., 2015).
Effects of Propofol alone or Combined with Curcumin on the Cell Viability of MN9D Cells
Propofol abuse can cause neurotoxicity and cognitive deficits. Autophagy dysregulation contributes to neuronal injury. Curcumin is a natural polyphenol derived from the turmeric plant, and it has neuroprotective and cognition-enhancing properties. He's team investigated whether curcumin protects MN9D dopaminergic cells against propofol-induced neurotoxicity by modulating autophagy via the Akt/mTOR/p70S6K signaling pathway.
They first evaluated MN9D cell cytotoxicity using CCK-8 assays. Methanol was used as a solvent and a negative control due to the hydrophobicity of curcumin. Cells were treated with 1-80 μM curcumin or 5-80 μg/mL propofol for 24 h (Fig.1c and d). Curcumin had low toxicity, even at 80 μM. Propofol was dose-dependently cytotoxic (viability was reduced to about 80% at 40 μg/mL) and decreased cell density. Therefore, 40 μg/mL propofol was used with 10-40 μM curcumin to explore neuroprotection (Fig. 1e and f). After 24 h, propofol-treated cells were smaller in size with decreased nodules, but curcumin co-treated cells had an almost 90% viability and exhibited protection from propofol-induced senescence with spindle-like morphology.
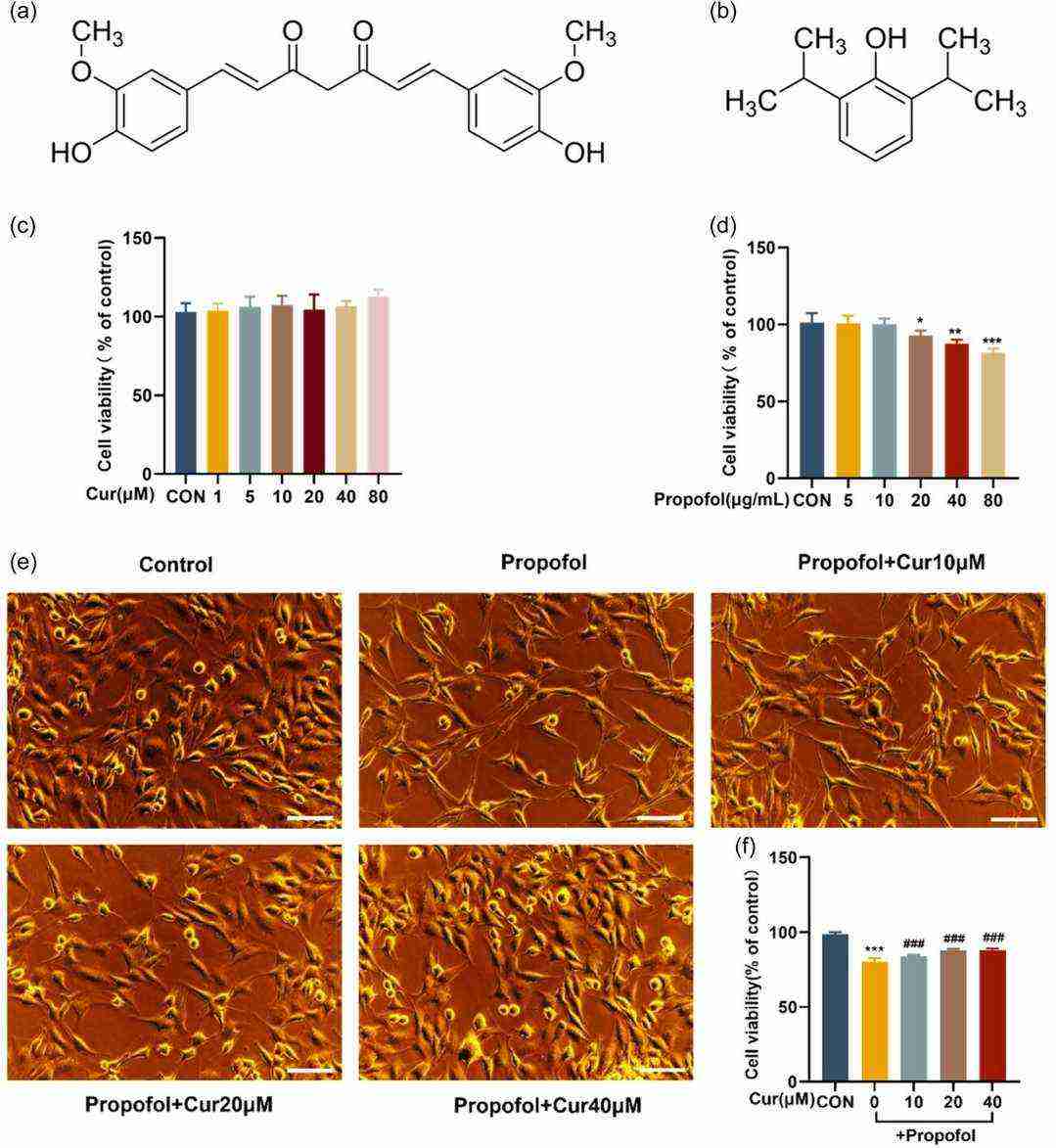 Fig. 1. Impact of propofol alone or in combination with curcumin on MN9D cell growth (He H X, Han Y P, et al., 2024).
Fig. 1. Impact of propofol alone or in combination with curcumin on MN9D cell growth (He H X, Han Y P, et al., 2024).
Rotenone and Antimycin A Significantly Reduced ATP Production and Disrupt Catecholamine Biosynthesis in MN9D Cells
Mitochondrial dysfunction is central to Parkinson's disease (PD), as dopaminergic neurons are highly susceptible to oxidative stress and energy depletion. Rotenone (Complex I inhibitor) and antimycin A (Complex III inhibitor) disrupt mitochondrial function, but their comparative effects on dopaminergic neurons remain unclear.
Adetuyi et al. investigated how rotenone and antimycin A induce energy depletion, oxidative stress, and neuronal death in MN9D dopaminergic cells to elucidate their roles in PD pathogenesis. Figure 2 demonstrates ATP levels in MN9D cells treated with mitochondrial toxins. Cells exposed to rotenone (1.5 µM) or antimycin A (10 µM) for 1 hour showed significantly reduced ATP production compared to control (KRB-HEPES buffer, pH 7.5), measured via colorimetric/fluorometric assays. To assess catecholamine biosynthesis, cells were treated with rotenone (1.5 µM), antimycin A (10 µM), or tetrabenazine (1 µM) for 1 hour. Figure 3a-c reveals that all three toxins markedly decreased dopamine levels versus control, as quantified by HPLC.
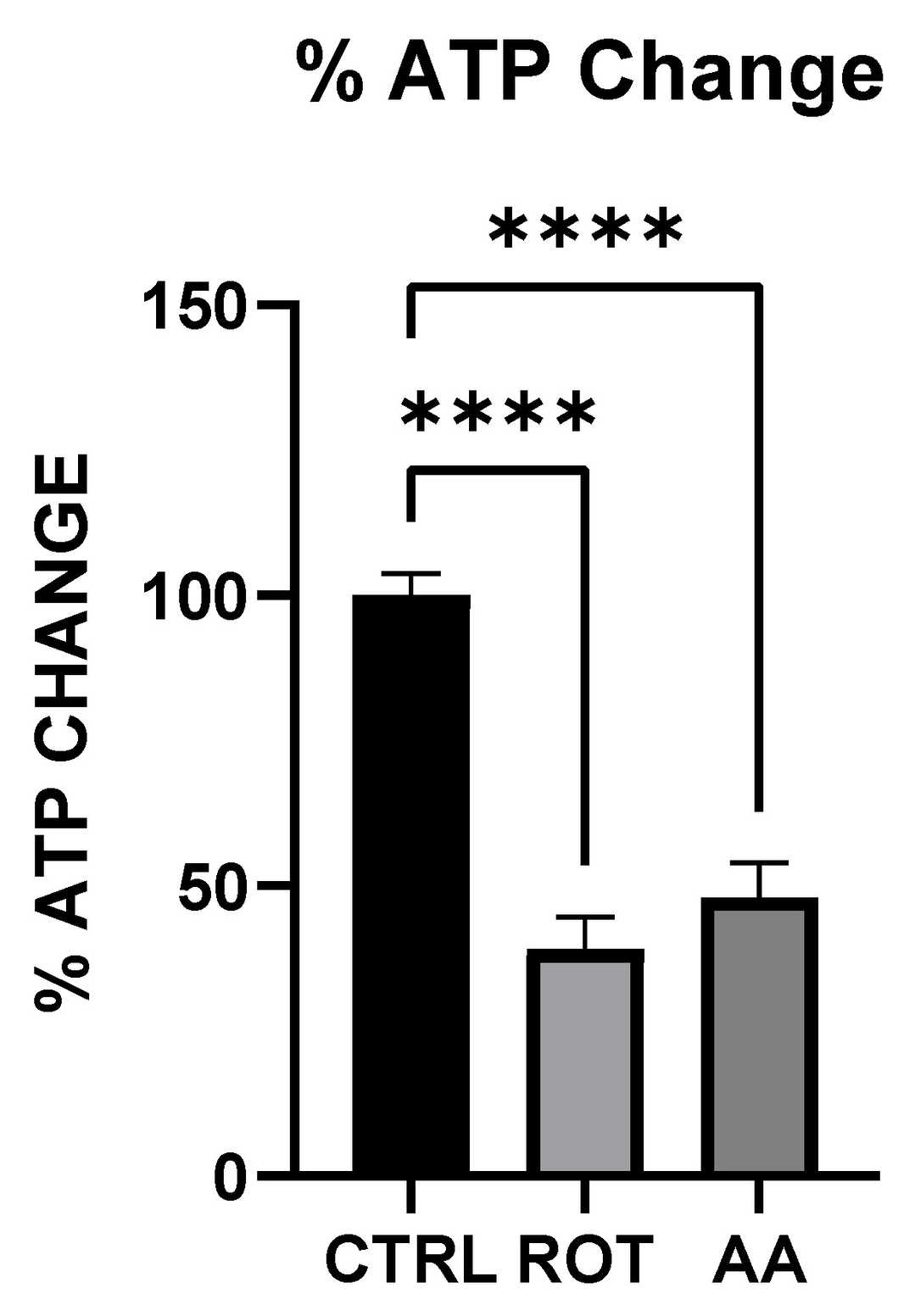 Fig. 2. Effect of Toxins treatment on ATP level (Adetuyi OA, Wimalasena K, et al., 2025).
Fig. 2. Effect of Toxins treatment on ATP level (Adetuyi OA, Wimalasena K, et al., 2025).
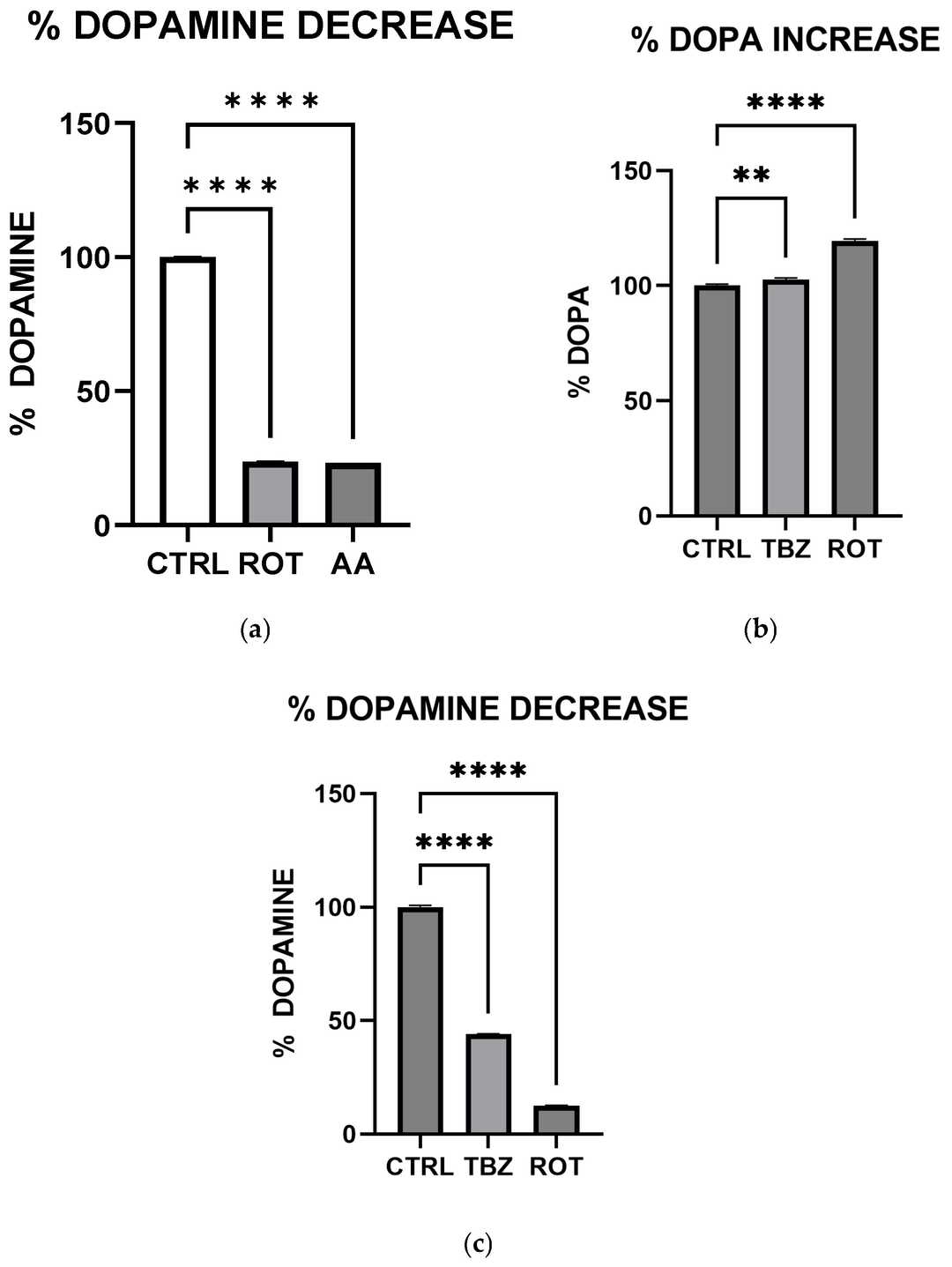 Fig. 3. Rotenone and Antimycin A disrupt catecholamine biosynthesis (Adetuyi OA, Wimalasena K, et al., 2025).
Fig. 3. Rotenone and Antimycin A disrupt catecholamine biosynthesis (Adetuyi OA, Wimalasena K, et al., 2025).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
