SK-MM-2
Cat.No.: CSC-C0465
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: round single cells growing in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD10 -, CD13 -, CD19 -, CD20 -, CD34 -, CD37 -, CD38 +, CD80 -, HLA-DR -, sm/cyIgG -, sm/cyIgM -, smkappa -, cykappa +, sm/cylambda -
Viruses: ELISA: r
SK‑MM‑2 is a human multiple‑myeloma (MM) cell line that was established from the bone‑marrow plasma cells of a male patient with plasma‑cell leukemia. The line grows exclusively in suspension culture, displays plasmacytoid morphology, and has a relatively slow doubling time of about 60 h. It is EBV‑negative and expresses the pan‑B‑cell marker B1 together with late B‑cell/plasma‑cell markers BL3, OKT10 and PCA‑1, while lacking T‑cell, myeloid or early B‑cell markers. Uniquely, SK‑MM‑2 secretes only κ light chains and no heavy chains, classifying it as a light‑chain‑only MM model. Genetically, the line harbors multiple copy‑number alterations: high‑level amplification of MALT1 (>10 copies), gains of c‑MYC and cyclin D1 (CCND1) mRNA over‑expression, and BCL2 amplification. Conventional karyotyping shows a highly aneuploid genome with recurrent 1q gains, 17p and 1p deletions.
Because of its light‑chain‑only phenotype and defined genetic lesions, SK‑MM‑2 is widely used for:
- Investigating Ig light‑chain regulation and the mechanisms underlying heavy‑chain silencing in MM.
- Studying the functional impact of MALT1, c‑MYC, BCL2 and cyclin D1 amplifications on proliferation and survival.
- Screening anti‑myeloma agents, especially BCL‑2 inhibitors such as venetoclax, where SK‑MM‑2 shows BCL‑2 dependence.
- Evaluating splice‑switching oligonucleotides targeting immunoglobulin variable exons for selective plasma‑cell killing.
- Analyzing the side‑population (SP) fraction and metabolic adaptations in the bone‑marrow microenvironment.
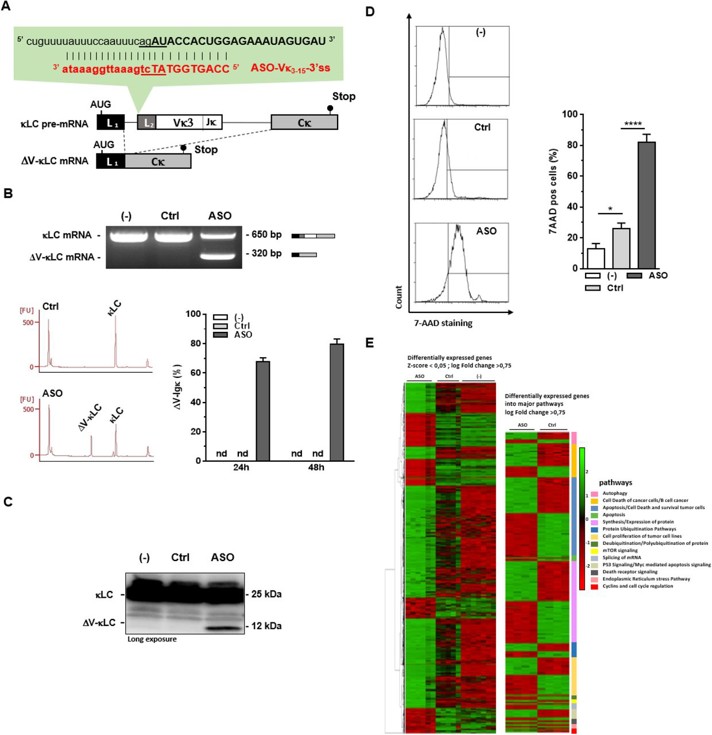
Impact Of ASO-Mediated Targeting of V Exon 3'ss in Myeloma Cells SK-MM-2
Systemic AL amyloidosis is caused by organ deposition of amyloid fibrils derived from monoclonal immunoglobulin light chains produced by a deregulated plasma-cell clone. Lambert et al. explored whether splice-switching antisense oligonucleotides (ASOs) directed against the variable (V) exon of the clone-specific pre-mRNA can force expression of a V-domain-less, truncated light chain that triggers ER stress-mediated apoptosis.
3' splice sites (3'ss) are more conserved than 5'ss, so ASOs targeting 3'ss excise exons more efficiently. In SK-MM-2 myeloma cells expressing IGKV3-15IGKJ4 κ light chain, ASO-Vκ3-15-3'ss (Fig. 1A) triggered near-complete V-exon skipping: ΔV-κLC mRNA rose to 67.7 ± 7.1 % (24 h) and 79.5 ± 9.0 % (48 h) of total Igκ transcripts (Fig. 1B). A ~12 kDa truncated ΔV-κLC protein appeared while full-length κ chains declined (Fig. 1C). ΔV-κLC accumulation coincided with increased cell death (Fig. 1D). RNA-seq (|log2FC| ≥ 0.75, adj p < 0.05) revealed 1 100 up- and 524 down-regulated genes enriched for apoptosis, autophagy, ubiquitination and ER-stress pathways, indicating that ER-stress-driven apoptosis underlies the massive lethality of ASO-Vκ3-15-3'ss (Fig. 1E).
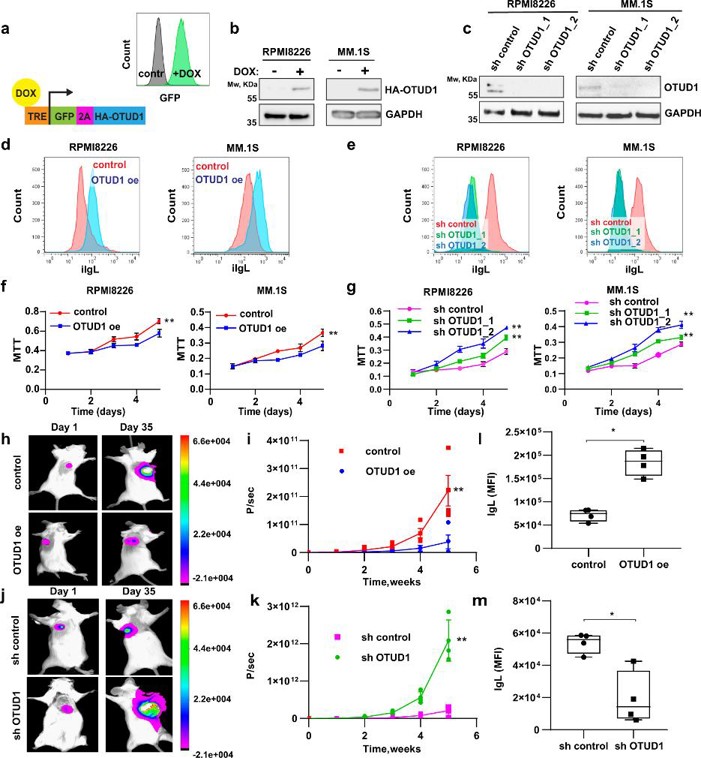
OTUD1 is a Regulator of Ig Production and Proliferation in Myeloma Cells
Serum Ig quantity fails to stratify MM prognosis; intracellular Ig load does. Integrating CRISPR screening with transcriptomic/proteomic data, Vdovin et al. identified OTUD1 as a deubiquitinase essential for Ig synthesis, proteasome-inhibitor (PI) sensitivity and tumor burden.
To probe OTUD1's role in Ig production, they generated MM lines (RPMI8226, MM.1 S, HEK 293, JJN-3, SK-MM-2, and U2OS) with dox-inducible OTUD1 overexpression (OTUD1 oe) or two shRNA knock-downs (sh OTUD1_1/2) (Fig. 2a-c); controls lacked dox or carried non-targeting shRNA. OTUD1 oe raised intracellular Ig light chain (iIgL) (Fig. 2d), whereas sh OTUD1 reduced it (Fig. 2e), without altering IgL mRNA or secretion. Knock-down of other DUBs had no effect, and catalytically dead OTUD1-C320R failed to change iIgL, confirming that OTUD1 enzymatic activity specifically sustains high IgL levels. Consistent with public data, OTUD1 curbed myeloma aggressiveness: OTUD1 oe slowed proliferation, whereas sh OTUD1 accelerated it (Fig. 2f, g). In vivo, the growth difference was magnified (Fig. 2h-k), and intracellular iIgL in xenograft tumors tracked OTUD1 levels (Fig. 2l, m). OTUD1 oe imposed a partial S-phase arrest without compromising viability, recapitulating patient observations and validating the model for dissecting OTUD1 function in myeloma.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells