REC-1
Cat.No.: CSC-C0005
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: small round cells growing singly or in clumps in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD10 -, CD13 -, CD19 +, CD20 +, CD34 -, CD37 +, CD38 +, cyCD79a +, CD80 +, CD138 -, HLA-DR -, sm/cyIgG -, sm/cy IgM +, sm/cykappa +, sm/cy lambda -
Viruses: PCR: EBV -, HBV -,
REC-1 is a human mantle cell lymphoma (MCL) cell line. The cell line was initially isolated from lymph node tissue obtained from a 61-year-old man with end-stage refractory B-cell non-Hodgkin lymphoma, blastoid variant, transformed, mantle cell lymphoma. Parental leukemic cells from the patient are found in lymph nodes, bone marrow, peripheral blood, and gastrointestinal lymphoid tissues in vivo, which are common sites of MCL. REC-1 cells are lymphoblastic in appearance in vitro: medium-sized cells with high nuclear to cytoplasmic ratio, prominent dense chromatin, and scant cytoplasm. The cells grow in suspension as single cells or loose cell aggregates.
REC-1 cells possess the t(11;14)(q13;q32) chromosomal translocation that leads to overexpression of Cyclin D1 (CCND1) and frequently have high-frequency mutations in lymphoma-related genes including ATM and TP53. The cell line retains malignant B-cell characteristics such as uncontrolled proliferation, resistance to apoptosis, and aberrant cell cycle regulation. REC-1 is used in research into MCL to understand the mechanisms that lead to B-cell malignant proliferation, cell cycle dysregulation and resistance to apoptosis. It is also used to test the efficacy of targeted small molecule therapies (such as BTK, BCL2 or mTOR inhibitors) and immunotherapies (such as anti-CD20 antibodies or CAR-T cells) as well as understand the mechanisms of drug resistance.
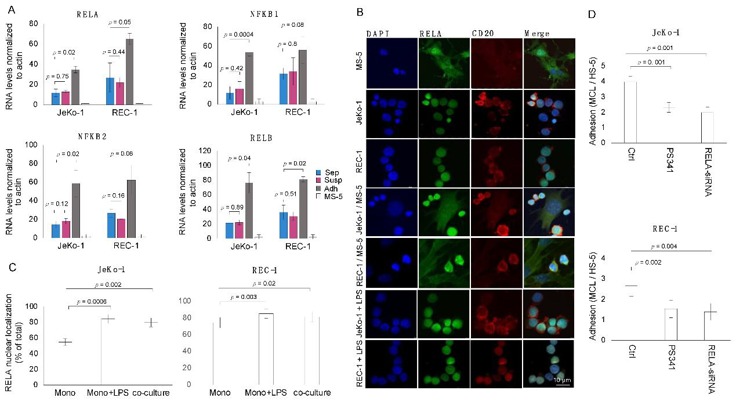
Adhesion of MCL Cells to Stromal Cells Is Associated with and Requires Induced NF-κB Activity
Mantle cell lymphoma (MCL) is a rare B-cell malignancy that traffics into lymph nodes and other organs. Stromal cells that compose the tumor microenvironment, provide a support system for MCL proliferation and drug resistance. Studies demonstrate frequent dysregulation of NF-κB signaling in MCL which is important for both MCL cell survival and adhesion. Sadeghi et al. aim to investigate the role of KDM6B histone demethylase in MCL cell adhesion to stromal cells and its potential as a therapeutic target.
MCL cells (JeKo-1 and REC-1) interact with stromal cells in microenvironments, leading to increased expression of NF-κB signature genes. To validate RNA-seq results, qPCR was used to measure transcript levels of four NF-κB components (NFκB1, NFκB2, RELA, RELB) in mono-cultured and co-cultured JeKo-1 and REC-1 cells (Fig. 1A). Increased transcript levels were observed in adherent JeKo-1 compared to suspension and mono-cultured JeKo-1 cells. Increased NF-κB gene expression in adherent cells was associated with increased nuclear RELA protein levels (Fig. 1B, C). Adherent JeKo-1 and REC-1 cells had significantly more RELA nuclear immunofluorescent staining than mono-cultured cells. RELA nuclear localization in adherent cells was near the level of mono-cultured cells treated with LPS (Fig. 1B, C). Activation of NF-κB is required for MCL cell adhesion to stromal cells. Inhibition of NF-κB activity using Bortezomib (PS341) or siRNA targeting RELA decreased MCL cell adherence (Fig. 1D).
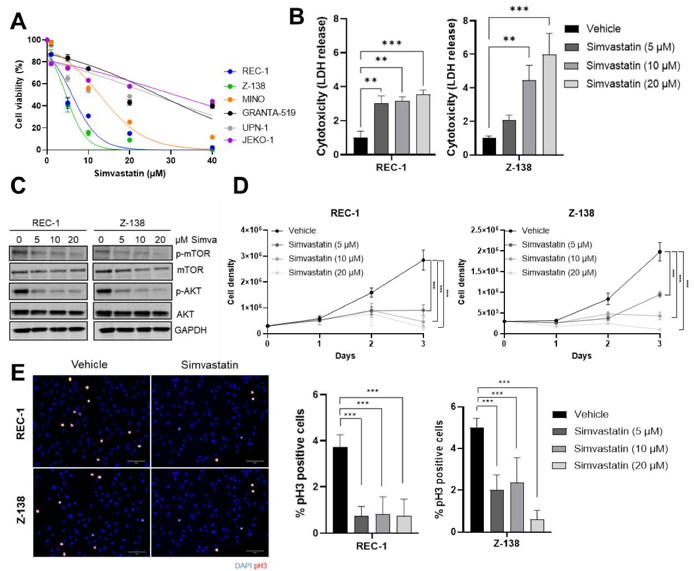
Simvastatin Impairs the Proliferation and Triggers a Caspase-Independent ROS-Mediated Cell Death of MCL Cells
Mantle cell lymphoma (MCL) is a rare and aggressive subtype of B-cell non-Hodgkin lymphoma that remains incurable with standard therapy. Statins, widely used as cholesterol-lowering agents, have shown anti-cancer activity through pleiotropic effects, including inducing lymphoma cell death. However, their activity as anti-MCL agents has not been tested. Santos et al. determined the activity of simvastatin on MCL cells.
To study the effect of simvastatin on MCL cell growth, they treated six MCL cell lines (REC-1, Z-138, JEKO-1, MINO, GRANTA-519, and UPN-1) with different concentrations. After 48 h, REC-1 and Z-138 were the most sensitive to simvastatin, showing IC50 values of 4.97 µM and 3.78 µM, respectively, while the remaining cell lines displayed higher IC50 values (Fig. 2A). Therefore, they selected REC-1 and Z-138 cell lines for the rest of the experiments. The addition of simvastatin at 5, 10, and 20 µM induced a 3-fold, 2.3-, 5.0-, and 6.7-fold increase in LDH release for REC-1 and Z-138, respectively, when compared with control (Fig. 2B). Santos et al. next studied the effect of simvastatin on PI3K/AKT/mTOR, a signaling cascade frequently deregulated in different cancers. The addition of simvastatin induced a strong reduction of the AKT and mTOR phosphorylation levels in REC-1 and Z-138 in a dose-dependent manner (Fig. 2C). Based on these results, they analyzed the effect of simvastatin on cell proliferation and mitotic index. Cell counting and phospho-H3 immunofluorescence revealed that simvastatin strongly inhibited cell proliferation and mitotic index in both cell lines (Fig. 2D, E). These results indicate that the antitumor activity of simvastatin could be a consequence of its ability to deregulate the PI3K/AKT/mTOR pathway and cell proliferation.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells