HBE135-E6E7
Cat.No.: CSC-C9185W
Species: Homo sapiens (Human)
Source: Lung; Bronchus
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The HBE135-E6E7 cell line is an immortalized human bronchial epithelial cell line that has been used in a variety of studies, including respiratory disease, inflammatory response, drug screening, and cell signaling pathway research. It was originally derived from normal bronchial epithelial cells of a male patient who underwent a lobectomy for squamous cell carcinoma and was later infected with recombinant retrovirus LXSN16E6E7 to establish the HBE135-E6E7 cell line, which is a transformed cell line. The cell line retains some typical epithelial cell properties, including keratin expression and cell polarity.
In respiratory disease research, the HBE135-E6E7 cell line has been used to model various conditions, such as asthma, allergic inflammation, and bronchitis. For example, in one study, the HBE135-E6E7 cell line was used as an in vitro model to investigate the effects of Cimicifugoside on inflammatory factors and epithelial barrier function in allergic asthma. It is also frequently used in drug screening and toxicity testing. A study utilized the HBE135-E6E7 cell line to explore how tea polyphenols influenced oxidative damage and induced apoptosis when cells were exposed to tobacco condensate. The HBE135-E6E7 cell line is also used in studies investigating cell signaling pathways. Researchers examined how the JAK2/STAT6 signaling pathway affects mucin MUC5B expression using the HBE135-E6E7 cell line.
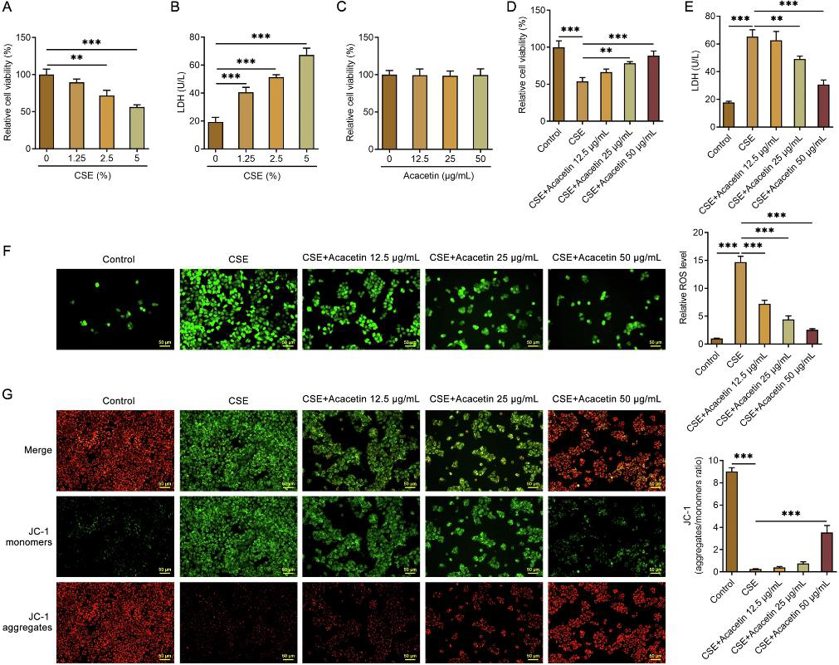
Acacetin Elevated Cell Viability and Alleviated Oxidative Stress Damage in CSE-Treated HBE135-E6E7 Cells
Chronic obstructive pulmonary disease (COPD) is a leading cause of death globally, with cigarette smoke being a major cause. Acacetin has shown lung-protective effects, but its role and mechanism in COPD are unclear. Chen's team explored how Acacetin protects human bronchial epithelial cells from cigarette smoke extract (CSE)-induced injury through the NRF2/SLC7A11/GPX4 signaling pathway.
They first investigated the effect of CSE on cell viability in HBE135-E6E7 cells. The results showed that CSE significantly reduced cell viability in a dose-dependent manner (Fig. 1A). Then the levels of LDH in the medium were measured as an injury index of cells. The results showed that LDH in the medium was increased in a dose-dependent manner by CSE (Fig. 1B). On this basis, 5% CSE was used in the following experiments. They then determined the influence of Acacetin on cell viability. As shown in Fig. 1C, Acacetin (12.5, 25, or 50 μg/ml) alone had no significant effect on cell viability. Acacetin significantly alleviated the decrease of cell viability caused by CSE in a dose-dependent manner (Fig. 1D). The results of LDH showed that Acacetin reduced LDH in the medium and suggested alleviation of cell injury (Fig. 1E). Acacetin decreased the generation of ROS in a dose-dependent manner (Fig. 1F). In addition, Acacetin attenuated CSE-induced MMP loss (Fig. 1G).
The IRE1α Inhibitor, 4μ8C, Exerts Suppressive Effects on the PM2.5-Induced Apoptosis of Airway Epithelial Cells
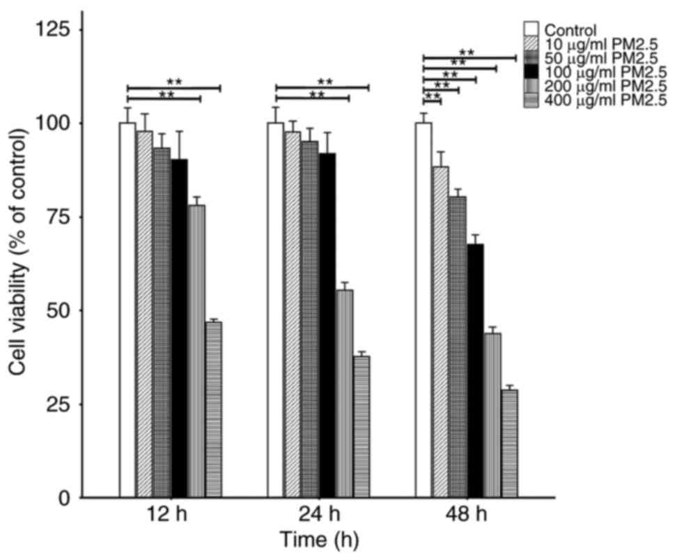
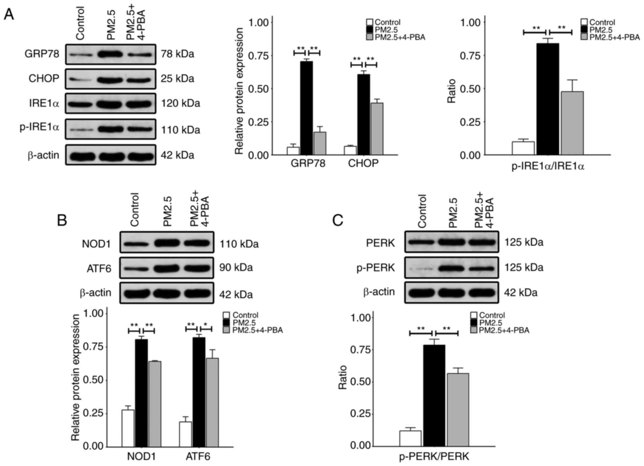
Fine particulate matter (PM2.5) is a major air pollutant that can cause airway inflammation. Hu's team investigated the effects of PM2.5 on endoplasmic reticulum (ER) stress and airway inflammation in human airway epithelial cells, specifically focusing on the role of the IRE1α/NOD1/NF‑κB pathway in these processes.
To identify the appropriate concentrations and time durations of PM2.5 for the study, HBE135-E6E7 cells were treated with different concentrations of PM2.5 for different time periods. The viability of cells was determined by CCK-8 assay. The viability analysis demonstrated no significant differences between the control and PM2.5-treated groups (10-100 μg/ml) at 12 and 24 hours but showed decreased viability in the 200 and 400 μg/ml groups from 12 to 48 hours and in 10-100 μg/ml groups at 48 hours. (Fig. 2). To explore whether PM2.5 can activate ER stress pathway, the expression of the proteins associated with ER stress was measured after PM2.5 treatment. Western blot was performed to detect the levels of proteins including GRP78, CHOP, p-IRE1α, NOD1, ATF6 and p-PERK. As shown in Fig. 3, exposure to 100 μg/ml PM2.5 for 24 h, significantly increased the expression of these proteins compared to the control group. However, 4-PBA, an ER stress inhibitor pre-treatment significantly inhibited the expression of these proteins compared to PM2.5 only treated group (Fig. 3).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells