FKH-1
Cat.No.: CSC-C0613
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: mostly single, round cells in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD4 +, CD11b +, CD13 +, CD14 -, CD15 +, CD19 -, CD33 +, HLA-DR +
Viruses: PCR: EBV -, HBV -, HCV -, HIV -, HTLV-I/II -, SMRV -
In 1993, researchers established the FKH-1 cell line from a 61-year-old male patient's peripheral blood who was suffering from Philadelphia chromosome-negative chronic myeloid leukemia (CML). The patient developed trilineage myelodysplasia which evolved into acute myeloid leukemia (AML M4) during the refractory stage of leukemia transformation. FKH-1 is a suspension cell line comprising single round cells in culture medium which researchers use to investigate molecular processes and drug reactions in AML. This cell line assists researchers in examining chromosomal irregularities as well as gene expression patterns and drug reaction profiles. It serves as a comparison tool alongside OCI-AML6 and Kasumi-1 cell lines to identify molecular distinctions between different AML subtypes. FH-1 demonstrates significant molecular pathways and gene expression controls, such as elevated MYC expression and stable β-actin levels to provide control benchmarks, which makes it a perfect system for studying AML pathogenesis and therapeutic targets.
DEK::NUP214-Expressing FKH-1 Cells Depend on XPO1 for Their Oncogenic Activity
DEK::NUP214 acute myeloid leukemia (AML) is a distinct subtype of AML characterized by poor prognosis and resistance to chemotherapy. The DEK::NUP214 fusion protein is implicated in adverse clinical outcomes and interacts with XPO1, a nuclear export protein involved in crucial cellular processes. Given XPO1's role in various cancers, its inhibition has the potential to counteract DEK::NUP214-associated malignancy.
Utilizing human AML models, Charles Cano et al. explored XPO1's role by deleting it in DEK::NUP214-positive cells and applying the XPO1 inhibitor eltanexor. They validated DEK::NUP214 and XPO1 expression in FKH-1 cells using established leukemia cell lines as controls. RT-qPCR and Western blot showed that only FKH-1 cells expressed DEK::NUP214, while XPO1 was similarly expressed in both lines (Fig. 1A, B). Sequencing confirmed the DEK::NUP214 breakpoint (Fig. 2A), and co-immunoprecipitation confirmed XPO1's interaction with DEK::NUP214 (Fig. 1C). They deleted XPO1 in FKH-1 cells and controls using CRISPR/CAS9. Post-nucleofection, annexin V+ cells increased over time in XPO1-depleted FKH-1 cells, reducing viability and growth rate (Fig. 1D-F). RT-qPCR and immunoblotting confirmed XPO1 knockdown (Fig. 2D and E). Immunofluorescence showed decreased XPO1 and DEK::NUP214 co-localization in XPO1-depleted cells (Fig. 2F). In control lines, XPO1 knockdown had minimal effects. Thus, DEK::NUP214-expressing FKH-1 cells depend on XPO1 for their oncogenic activity.
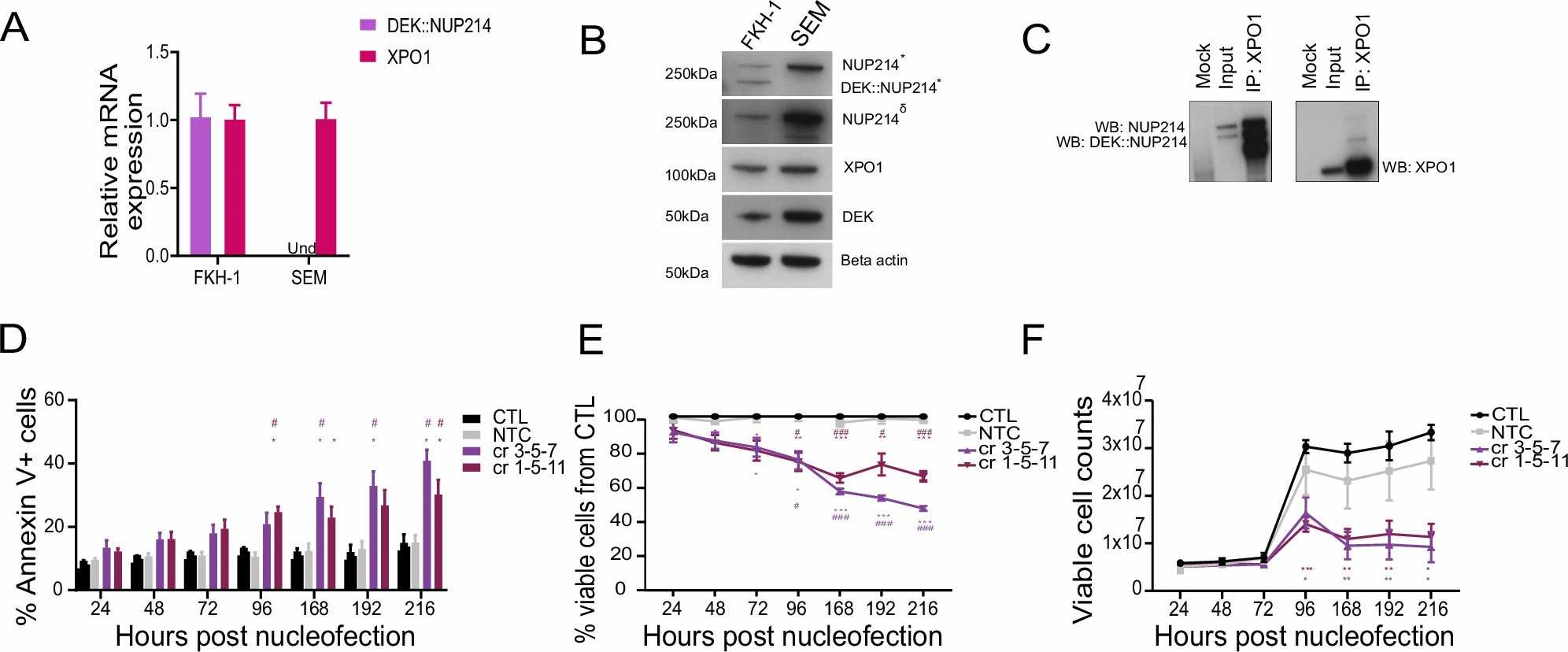 Fig. 1. DEK::NUP214 leukemia depends on XPO1 (Charles Cano F, Kloos A, et al., 2025).
Fig. 1. DEK::NUP214 leukemia depends on XPO1 (Charles Cano F, Kloos A, et al., 2025).
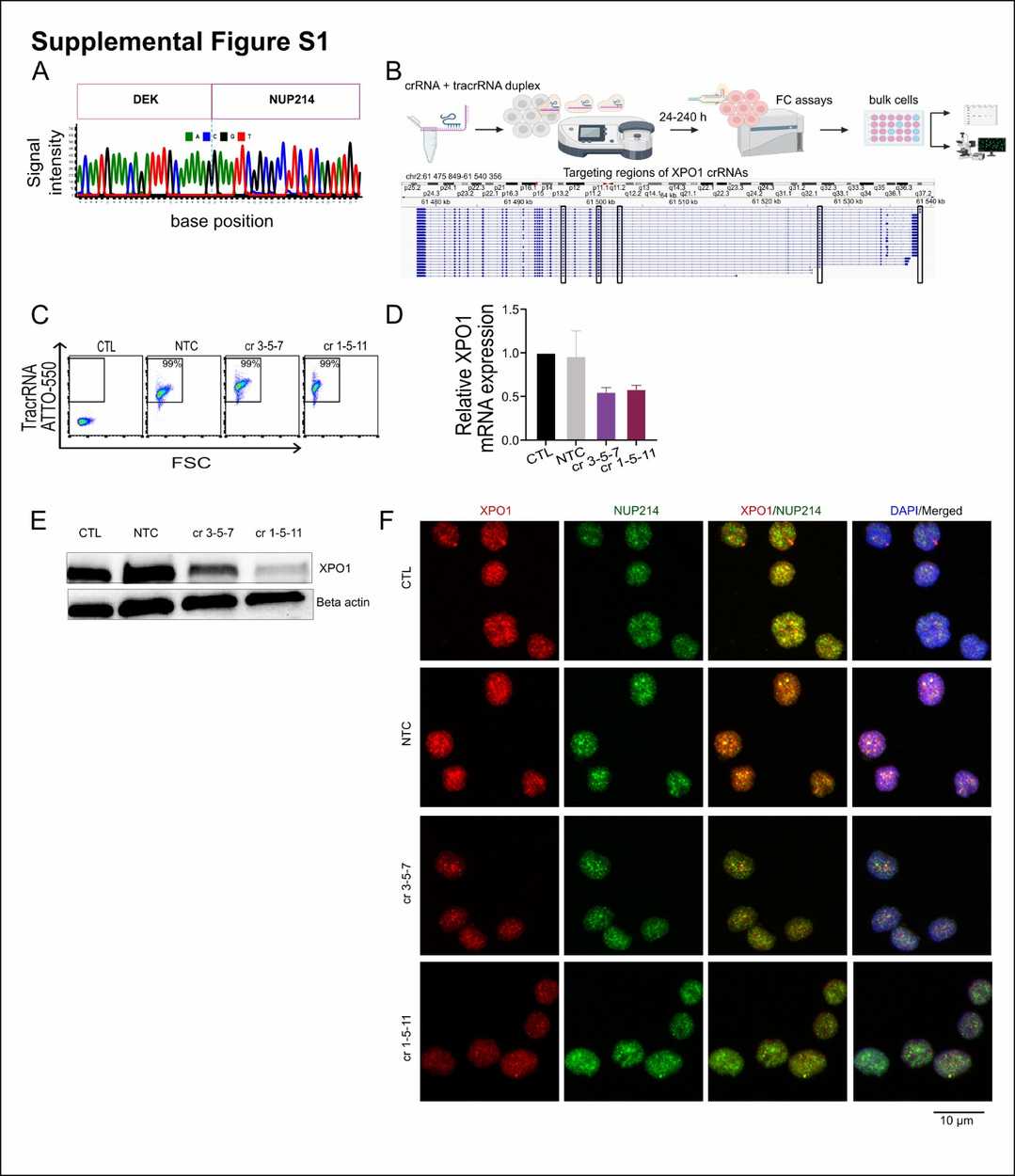 Fig. 2. Generation of XPO1 knockout in FKH-1 cells (Charles Cano F, Kloos A, et al., 2025).
Fig. 2. Generation of XPO1 knockout in FKH-1 cells (Charles Cano F, Kloos A, et al., 2025).
Leukemogenic NUP214 Fusion Proteins Locate to Nuclear Bodies in Patient-Derived Cells
Acute leukemia cases frequently display chromosomal translocations that produce SET-NUP214 and DEK-NUP214 fusion proteins from NUP214 which result in poor patient prognoses and resistance to treatment. CRM1 becomes a key player in leucemogenic processes because these fusion proteins disrupt nuclear export through CRM1. Mendes et al. aim to evaluate the potential of CRM1 as a therapeutic target in NUP214-related leukemia.
Using leukemia cell lines with endogenous SET-NUP214 and DEK-NUP214, they investigated the effects of CRM1 inhibition with LMB and SINE KPT-185 on protein localization and leukemia cell proliferation and viability. First, they examined the localization of NUP214 fusion proteins in various leukemia cell lines using anti-NUP214 antibodies and immunofluorescence microscopy. SET-NUP214 was found at the nuclear rim and in nuclear bodies in both LOUCY and MEGAL cells (Fig. 3A). In contrast, FKH-1 cells showed DEK-NUP214 in smaller nuclear bodies (Fig. 3A). GFP-tagged SET-NUP214 and DEK-NUP214 exhibited similar localizations in HCT-116 cells (Fig. 3B). In FKH-1 cells, NUP214 antibodies also marked the nuclear rim, likely indicating endogenous NUP214 rather than the fusion protein. OCI-AML1 and MOLM-13 cells showed typical nucleoporin patterns at the nuclear rim, as they do not express NUP214 fusions (Fig. 3A). To explore FG repeat cohesion in SET-NUP214 body formation, LOUCY cells were treated with 5% 1,6-hexanediol, disrupting nuclear bodies within 10 minutes, illustrating their dependence on FG repeat interactions (Fig. 3C).
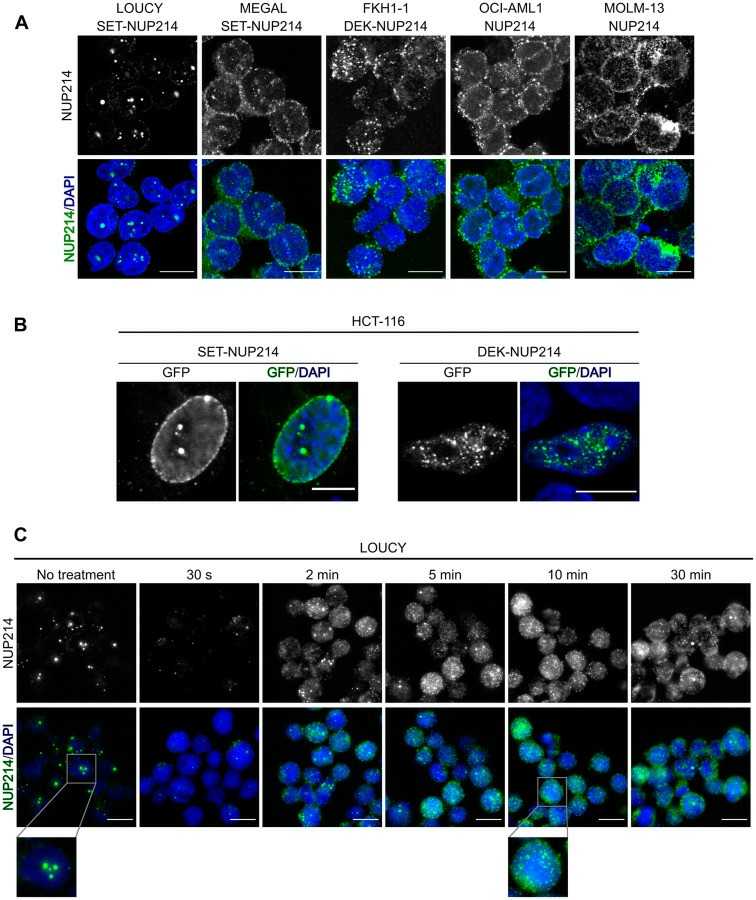 Fig. 3. NUP214 fusion proteins localize to distinct nuclear bodies (Mendes A, Jühlen R, et al., 2020).
Fig. 3. NUP214 fusion proteins localize to distinct nuclear bodies (Mendes A, Jühlen R, et al., 2020).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells