ZEM2S
Cat.No.: CSC-C9147W
Species: Danio rerio (Zebrafish)
Source: Blastula
Morphology: fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
ZEM2S is a fully characterized, commercially available fibroblast-like cell line, derived from zebrafish (Danio rerio) embryos. It was created in 1993, derived from the original ZEM2 cell line, that was cultured in a complex growth medium that was supplemented with insulin, trout embryo extract, and sera. ZEM2S cells have an adherent, fibroblast-like morphology and can be propagated in vitro over long periods of time. ZEM2S serves as a vital in vitro tool for a variety of research applications:
Toxicology and Ecotoxicology: The ZEM2S cell line is often used to screen the cytotoxicity/biological activity of environmental contaminants, pharmaceuticals, and chemicals in aquatic toxicology assays.
Gene Regulation Studies: As a cell-based model system, ZEM2S can be used to study the regulation of gene expression, and the differential activity of enhancers and promoters that are present during early zebrafish development.
3D Cell Culture and Mechanistic Studies: ZEM2S has been used successfully to create 3D spheroid cultures, which can provide a more physiologically relevant model for examining intercellular interactions, compound penetration, and modes of action/mechanisms of toxicity.
Cell Differentiation and Extracellular Signaling: ZEM2S can also be used as an in vitro assay system to study the extracellular factors that induce and modulate cellular differentiation processes.
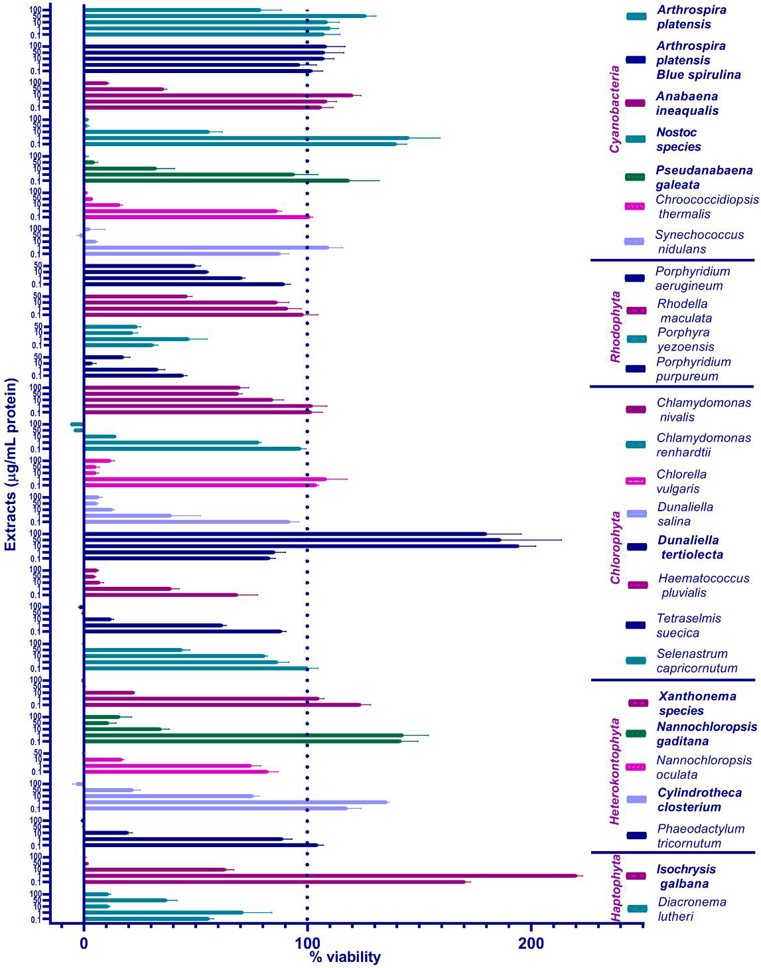
Primary Screening of Algal/Cyanobacterial Extracts in ZEM2S Fibroblasts-Testing Extracts' Cytotoxicity
Cultured meat production, currently dependent on expensive and environmentally harmful fetal bovine serum (FBS), needs sustainable alternatives for commercial viability. Sibinčić et al. evaluated twenty-six water-based algal and cyanobacterial extracts for their ability to support cell growth in reduced serum conditions. The extracts were initially tested in zebrafish cells (ZEM2S) with 1% FBS, and ten promising ones were further assessed in Japanese quail myoblasts.
The primary screening aimed to identify which algae/cyanobacteria and concentrations promote ZEM2S cell viability. They focused on the protein component of the extracts and used protein concentration to quantify the amount added to the cells. ZEM2S cells were maintained in 10% FBS-supplemented media. For the experiment, cells were seeded in 96-well plates in 1% FBS media with or without extracts. The cells were incubated with the extracts for 72 hours to assess long-term tolerance. They used the resazurin reduction (Alamar Blue) assay due to its higher sensitivity and because ZEM2S cells did not metabolize tetrazolium salts. Twenty-six extracts were tested: seven from cyanobacteria, four from Rhodophyta, eight from Chlorophyta, five from Heterokontophyta, and two from Haptophyta. Figure 1 shows the results: cell viability generally decreased at concentrations ≥ 10 μg/mL, especially at 50 and 100 μg/mL. However, Arthrospira platensis and Dunaliella tertiolecta showed better viability even at higher concentrations. The phycocyanin-enriched blue spirulina extract was better tolerated at 100 μg/mL than the full Arthrospira platensis extract. Dunaliella tertiolecta was particularly interesting as higher concentrations enhanced cell viability.
Prediction of Acute Fish Toxicity and Fish Embryo Toxicity Tests by Cytotoxicity Assays Using Liver and Embryo Zebrafish Cell Lines (ZFL And ZEM2S)
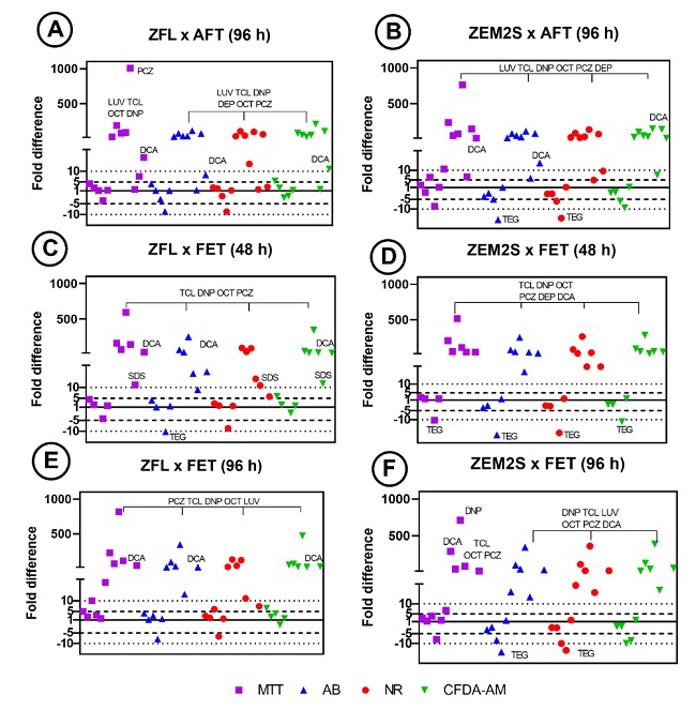
Fish cell-based assays offer potential alternatives to using vertebrates in ecotoxicology. This study assessed the cytotoxicity of thirteen chemicals in two zebrafish cell lines (ZEM2S and ZFL) to determine if IC50 values from viability assays (alamar blue, MTT, CFDA-AM, and neutral red) can predict LC50 values of Acute Fish Toxicity (AFT) and Fish Embryo Toxicity (FET) tests.
IC50 values showed both cell lines were significantly less sensitive (IC50 > 10-fold higher than the LC50) than AFT test in up to seven substances (i.e., PCZ, LUV, DNP, TCL, OCT, DEP (except in MTT assay), and DCA (except in NR assay)) (Figs. 2A and B). When assessed by ZFL cell line, the same substances, and additionally SDS, had IC50 values 10-fold higher than the LC50 of FET (48 h) (Fig. 2C). ZEM2S cell line was also significantly less sensitive than the FET (48 h) to the same substances (Fig. 2D). The fold differences of LUV, DEP and MQ were not calculated because no data is available for these chemicals in FET test (48 h). Considering the FET test (96 h), both cell lines were significantly less sensitive to PCZ, TCL, DNP, OCT LUV (except in MTT assay in ZEM2S), and DCA (except in NR in ZFL) (Figs. 2E and F). The fold differences of DEP and SDS were not calculated because there is no available data (LC50) for these substances in the FET test (96 h). On the other hand, ZEM2S was 10-fold more sensitive to TEG than AFT and FET (48 and 96 h) tests (Figs. 2B, D, and F). ZFL was also 10-fold more sensitive to TEG than the FET test (48 h) (Fig. 2C).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells