ML-1
Cat.No.: CSC-C0493
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: Lymphoblastoid
Culture Properties: Suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
CSF1PO: 10,11
D13S317: 9,12
D16S539: 9,12
D5S818: 12
D7S820: 9,11
THO1: 7,9.3
TPOX: 8,10
vWA: 16
ML-1 is a human acute myeloid leukemia (AML) cell line isolated from the peripheral blood of a 24-year-old patient. ML-1 cells are a suspension growth type cell line. The cell morphology is a typical leukemia-like cell morphology. The main features of leukemia-like cell morphology are larger cell volume and irregular nuclei.
The ML-1 cell line has been used in the research of tumor biology, cellular signal transduction and drug screening. It has been reported that ML-1 is sensitive to DNA damage and depolymerization inhibitors. The Hsp90 inhibitor Geldanamycin (GM) can alleviate G2 arrest in p53-negative leukemia cells through the down-regulation of Chk1. In addition, ML-1 cells are also used to study the mechanisms of leukemia cell differentiation and drug resistance. Furthermore, immunological studies use ML-1 cells to understand cytokine functions. For example, the expression of ML-1 gene is closely related to allergic reactions in asthmatic patients. The newly identified cytokine encoded by ML-1 gene is expressed in activated PBMCs, CD4+ T cells, mast cells and other cells. The cytokines secreted by ML-1 cells can induce the expression of IL-6 and IL-8, as well as up-regulate the expression of ICAM-1, thereby participating in the inflammatory response.
The Effect of 2-Deoxy-D-glucose on Glycolytic Metabolism in Acute Myeloblastic Leukemic ML-1 cells
Acute myeloblastic leukemia (AML) is one of the most frequent and most aggressive types of cancer, with high heterogeneity and metabolic plasticity. Developing new anti-cancer drugs by targeting metabolic pathways presents an innovative treatment approach. In this study, Christensen et al. used hyperpolarized 13C NMR spectroscopy, biochemical assays, and respirometry to examine the effects of 2-deoxy-D-glucose (2-DG) on the AML cell line ML-1. The study assessed the potential therapeutic utility of 2-DG in AML to suppress glycolysis and improve chemosensitivity. The proliferation of ML-1 cells was measured through trypan blue exclusion testing across different 2-DG concentrations and exposure periods (Fig. 1). No significant differences were found at 6 h, while two-way ANOVA indicated that cell growth was significantly inhibited at 5 mM after 24 h and at 2 mM after 48 h. Fig. 1B shows the cell growth at 0, 2, and 5 mM after 24 h. Those concentrations and exposure time were applied for all subsequent experiments because they were not too toxic but led to measurable effects. Cell viability (live/dead cell ratio) is presented in Fig. 1C. One-way ANOVA did not show significant differences between treatments.
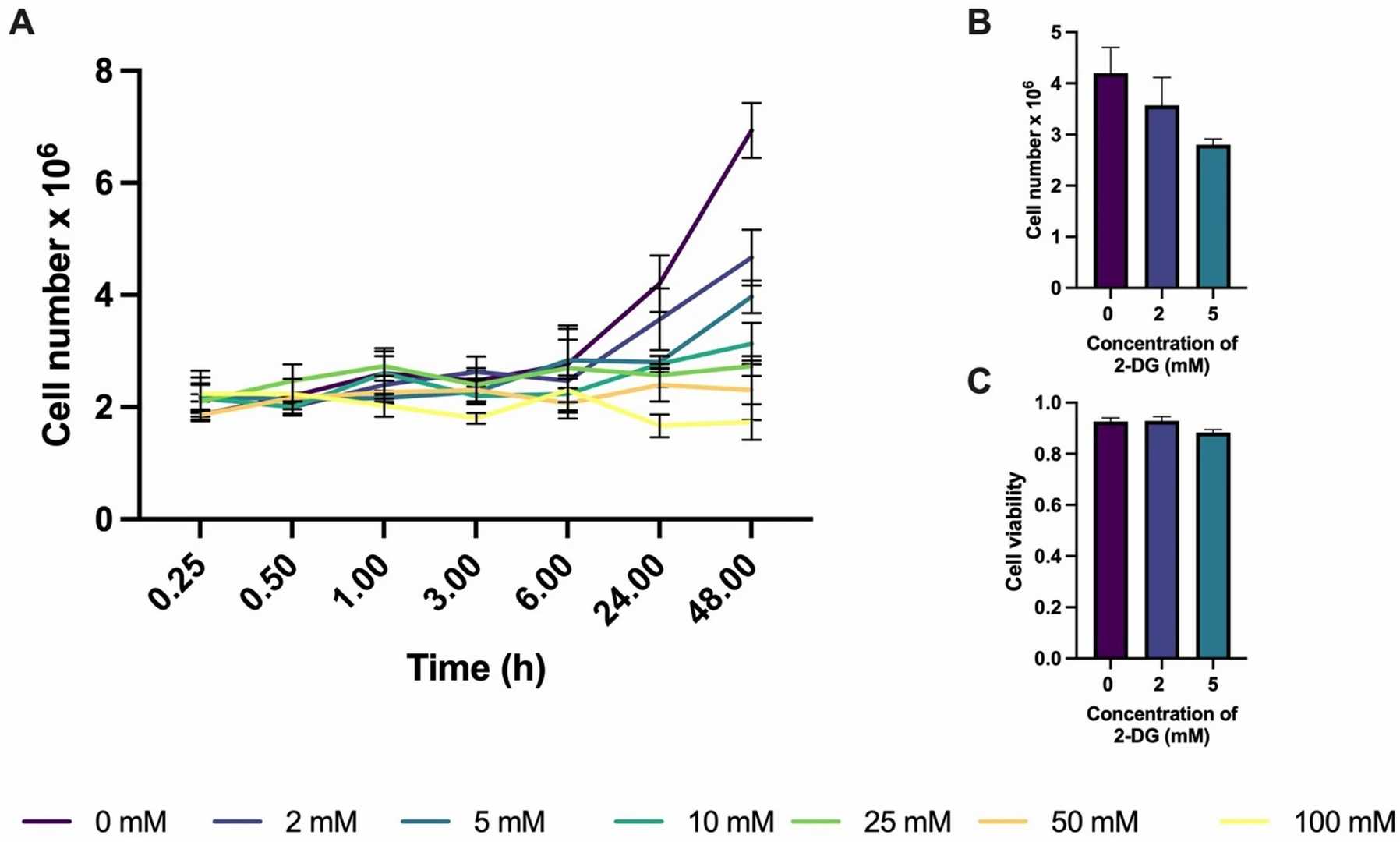 Fig. 1. Cell proliferation as a function of 2-DG treatment duration at various concentrations (Christensen N V, Knudsen J H, et al., 2025).
Fig. 1. Cell proliferation as a function of 2-DG treatment duration at various concentrations (Christensen N V, Knudsen J H, et al., 2025).
Effect on Cell Viability of ML-1 When Treated with CdSe/ZnS and InP/ZnS QDs
Photostable semiconductor nanocrystals called quantum dots (QDs) serve important roles in medical imaging for cancer and therapeutic applications. However, the interactions of QDs with cancer cells, including uptake and intracellular trafficking kinetics, are not well understood. Zhang's team studied the cytotoxicity and intracellular transport kinetics of CdSe/ZnS and InP/ZnS QDs in ML-1 thyroid cancer cells with HeLa cells as control. Cells were treated with QDs at various concentrations of 4.6 to 167 µg/mL for 24 h. After 7 h of incubation with XTT reagent, the viability of the cells was determined. As shown in Fig. 2A and B, no viability defect in ML-1 thyroid cells was observed. In contrast, the viability of HeLa cells decreased by ~30% with 167 µg/mL green CdSe/ZnS QDs (Fig. 2C). Red Cd QDs had no effect on HeLa cells (Fig. 2C). Green InP/ZnS QDs decreased the viability of HeLa cells by ~40% at 167 µg/mL, and red InP/ZnS QDs decreased the viability by ~30% (Fig. 2D). As a positive control, the cells were exposed to 5–20% DMSO (Fig. 2). Mouse fibroblast cells were also evaluated (Fig. 2E and F). Minor viability differences were noticed among all treatment groups. However, Cd QDs were slightly more toxic than InP QDs. Overall, HeLa cells were more sensitive to both Cd and InP QDs than ML-1 cells. This is evident from a significant decrease in HeLa cell viability.
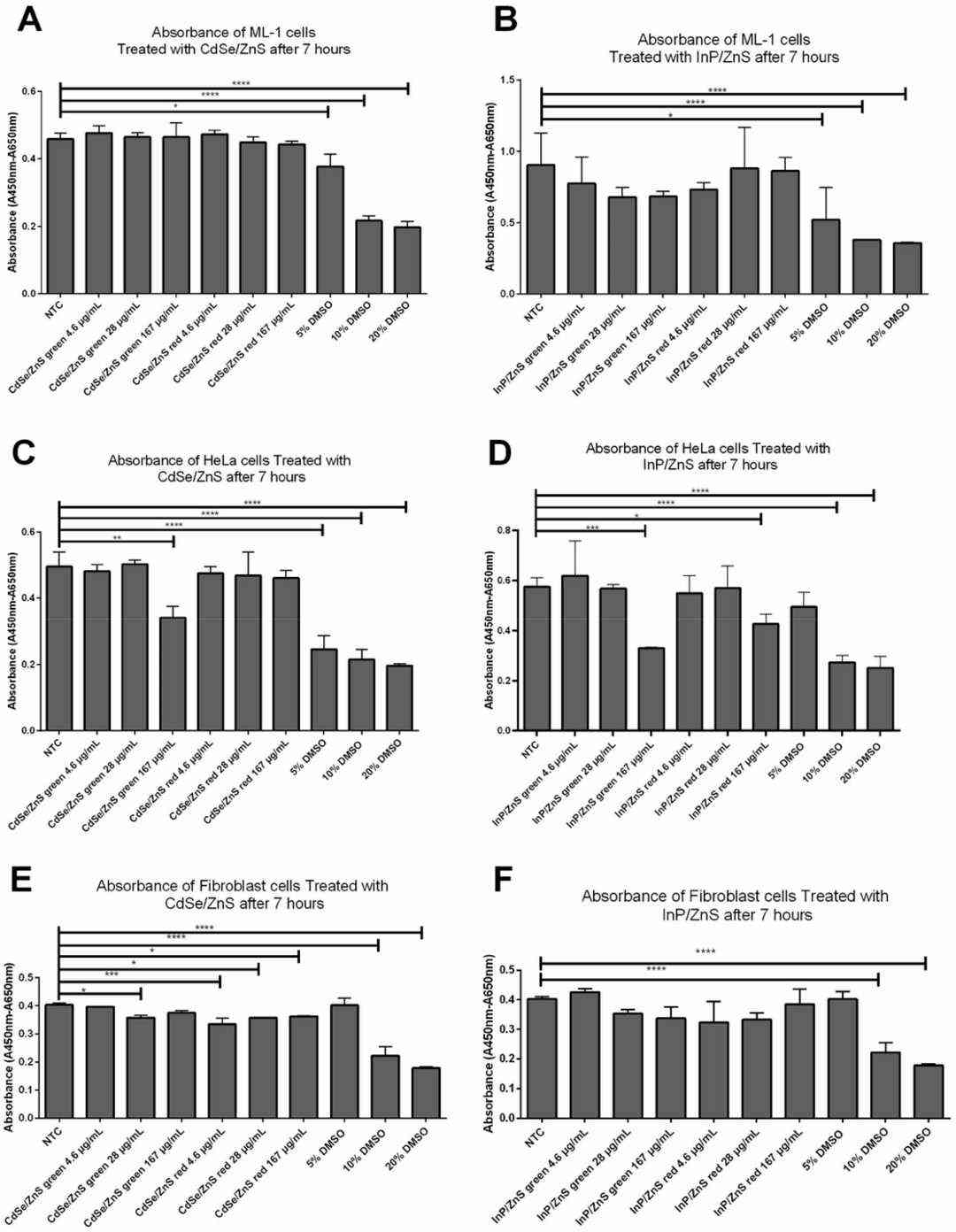 Fig. 2. Effects of CdSe/ZnS and InP/ZnS QDs on cell viability in ML-1 and HeLa cell lines measured by XTT reagents (Zhang M, Kim DS, et al., 2022).
Fig. 2. Effects of CdSe/ZnS and InP/ZnS QDs on cell viability in ML-1 and HeLa cell lines measured by XTT reagents (Zhang M, Kim DS, et al., 2022).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells