MKN-1
Cat.No.: CSC-C9493L
Species: Homo sapiens (Human)
Source: Lymph Node Metastasis
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
vWA: 16
FGA: 20,23
Amelogenin: X
TH01: 9
TPOX: 8
CSF1P0: 9,12
D5S818: 11
D13S317: 10,12
D7S820: 10
Shipping Condition: Room Temperature
Gastric adenocarcinomas, the predominant histological subtype of gastric cancer, account for the majority of stomach cancer cases globally. The MKN-1 cell line provides a valuable in vitro platform to study the molecular mechanisms and pathways that drive the development and progression of this disease, which often presents at advanced stages and is associated with poor prognosis.
Originating from an elderly male patient of Mongoloid ancestry, the MKN-1 cell line offers a unique perspective on the genetic and phenotypic characteristics of gastric tumors that may be influenced by factors such as age, sex, and ethnicity. This diversity in the cancer cell models available is crucial for gaining a comprehensive understanding of the heterogeneity observed in gastric cancer, which can ultimately inform the development of more personalized treatment strategies.
Extensive research has been conducted using the MKN-1 cell line, with studies focusing on areas such as cell signaling, drug sensitivity, and the identification of novel therapeutic targets. The well-characterized nature of this cell line has made it a widely used and reliable model system in the field of gastric cancer research.
Inhibition of Cell Proliferation by Enforced Expression of RUNX3 in MKN-1 Cells
The human runt-related transcription factor 3 gene (RUNX3) is considered to be a candidate tumor suppressor gene in gastric carcinoma. However, the role of RUNX3 in the regulation of cell proliferation remains unclear. An MTT assay was carried out to examine whether enforced expression of RUNX3 could suppress cell proliferation. The viability of the cells demonstrated significant inhibition of proliferation in MK N-1 cells transduced with RUNX3 in a time-dependent manner, compared to LacZ-transduced or non-transduced cells (Fig. 1a; P < 0.001). MKN-1 cells co-infected with Ad-Tet-On and Ad-Tet-LacZ or non-infected cells did not show growth inhibition even in the presence of Dox (Fig. 1a). In the case without Dox treatment, the growth rate of RUNX3-transduced cells did not differ significantly from that of LacZ-transduced or non-transduced cells as controls (Fig. 1b). These results suggest that RUNX3 acted as a suppressor of cell proliferation in MKN-1.
Flow cytometry revealed that the percentage of cells with a sub-G1 peak, indicative of apoptosis, was as high as 34.11%, compared with 7.05% in LacZ-transduced cells. The cell percentages in the G0/G1 phase, S phase, and G2/M phase were 31.91, 10.45, and 23.53% in the RUNX3-transduced cells, and 48.07, 8.66 and 36.22% in the LacZ-transduced cells, respectively (Fig. 2a). These data indicated that apoptosis was induced by enforced expression of RUNX3 in the MKN-1 cells. Then, apoptosis was also confirmed morphologically by Hoechst 33258 staining by RUNX3 expression in MKN-1 (Fig. 2b). In addition, cytochrome c release from the mitochondria into the cytosolic fraction and appearance of cleaved caspase-3 was observed in RUNX3-transduced cells (Fig. 2c, d). These results suggest that enforced expression of RUNX3 inhibited cell proliferation by inducing apoptosis via a mitochondria-mediated pathway in MKN-1 cells.
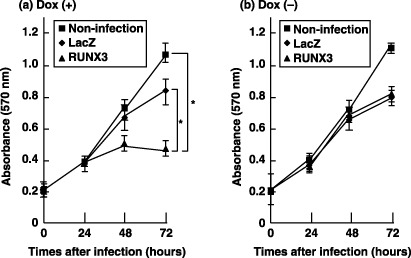 Fig. 1 Growth inhibition by runt-related transcription factor 3 (RUNX3) as determined using the MTT assay. (Nagahama Y, et al., 2007)
Fig. 1 Growth inhibition by runt-related transcription factor 3 (RUNX3) as determined using the MTT assay. (Nagahama Y, et al., 2007)
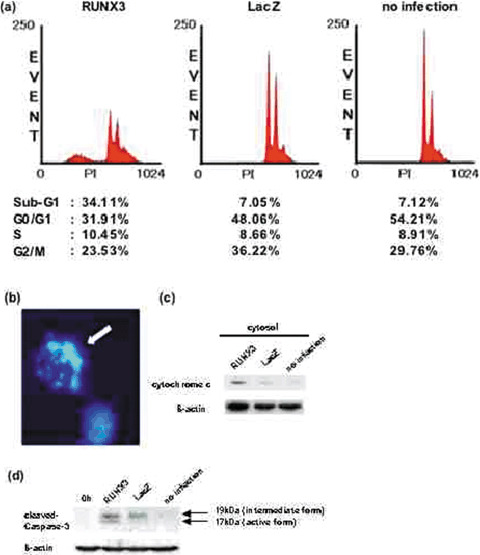 Fig. 2 Detection of apoptotic cells. MKN-1 cells were co-infected at a multiplicity of infection (MOI) of 40 (Ad-Tet-On, MOI 20; Ad-Tet-LacZ or Ad-Tet-FLAG-RUNX3, MOI 20) or left uninfected, and cultured with doxycycline (Dox) (1 µg/mL). (Nagahama Y, et al., 2007)
Fig. 2 Detection of apoptotic cells. MKN-1 cells were co-infected at a multiplicity of infection (MOI) of 40 (Ad-Tet-On, MOI 20; Ad-Tet-LacZ or Ad-Tet-FLAG-RUNX3, MOI 20) or left uninfected, and cultured with doxycycline (Dox) (1 µg/mL). (Nagahama Y, et al., 2007)
Effect of Trypsin on Adhesion of MKN-1 Cells to Various ECM Proteins
MKN-1 human gastric carcinoma cells transfected with trypsinogen-1 cDNA grow faster and adhere to fibronectin substrate more efficiently than the parent MKN-1 cells. The effect of trypsin treatment on adhesion of the parent MKN-1 cells was examined, which secrete neither trypsinogen nor trypsin, to a plastic plate precoated with various concentrations of the cell adhesion proteins fibronectin. MKN-1 cells were harvested by incubating with EDTA in the presence or absence of 6.25 μm trypsin for 10 min. After inactivation of trypsin with soybean trypsin inhibitor, cells were incubated on the fibronectin-coated plastic plate at 37°C for 1 h. Trypsin treatment increased the cell adhesion to fibronectin substrate 2-4-fold at low fibronectin concentrations (0.6-1.2 μg/ml) (Fig. 3). The trypsin-treated cells more rapidly spread on the fibronectin substrate than the control cells (Fig. 4). Trypsin treatment at 12.5 μm showed almost the same effect as that at 6.25 μm. On the other hand, trypsin treatment at 6.25 μm rather decreased the cell adhesion to vitronectin, type IV collagen, and laminin-1.
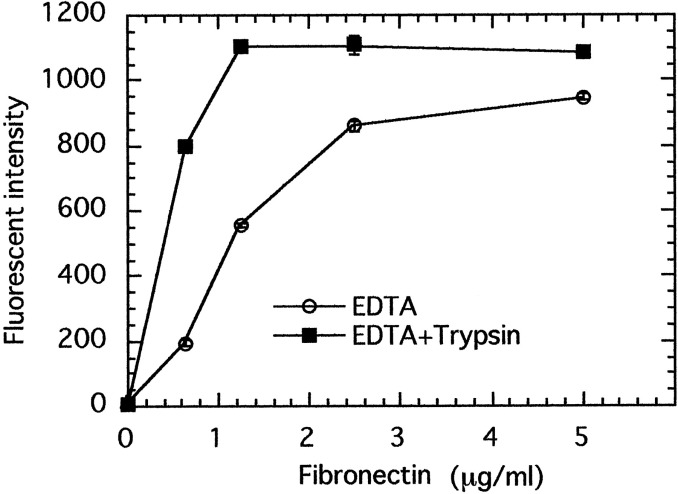 Fig. 3 Effect of trypsin treatment on adhesion of MKN-1 cells to fibronectin. (Miyata S, et al., 2000)
Fig. 3 Effect of trypsin treatment on adhesion of MKN-1 cells to fibronectin. (Miyata S, et al., 2000)
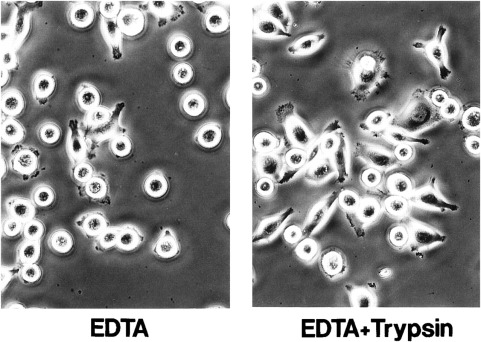 Fig. 4 Morphology of MKN-1 cells harvested with EDTA alone or with EDTA plus trypsin on fibronectin substrate. (Miyata S, et al., 2000)
Fig. 4 Morphology of MKN-1 cells harvested with EDTA alone or with EDTA plus trypsin on fibronectin substrate. (Miyata S, et al., 2000)
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells