HUT 102
Cat.No.: CSC-C9106W
Species: Homo sapiens (Human)
Source: Lymph Node
Morphology: lymphoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HUT‑102, also known as HuT‑102, is a suspension‑grown human T‑cell lymphoma cell line, originally established from the peripheral blood of a 26‑year‑old male patient with T‑cell lymphoblastic lymphoma. The cells are lymphoblast‑like and grow as clusters in suspension rather than adherent to the culture vessels. HUT‑102 cells can be maintained in RPMI‑1640 supplemented with 10 % fetal bovine serum, 100–200 U/mL recombinant IL‑2, and antibiotics at 37 °C in a humidified 5 % CO₂ incubator; population doubling time is ~38 hours. The HUT‑102 cell line expresses high levels of IL‑2 receptor while naturally harboring human T‑cell leukemia virus type I (HTLV‑I) which makes it a common model system for research on HTLV‑I‑induced oncogenesis and adult T‑cell leukemia/lymphoma (ATLL). HUT‑102 is commonly used for immunologic studies, viral replication assays, drug‑screening and gene‑editing studies. The well‑characterized phenotype, stable growth and availability of STR authentication data make HUT‑102 a useful resource for studying T‑cell signaling, tumor biology, and therapeutic target validation.
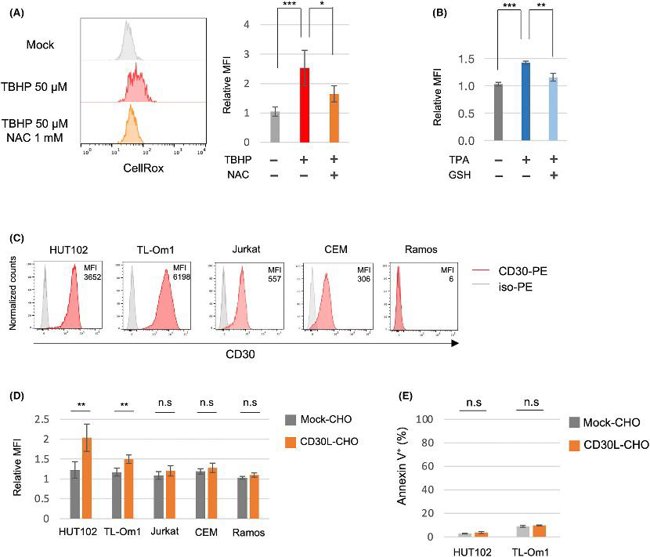
CD30 Signaling Induces an Increase of Intracellular Reactive Oxygen Species Without Inducing Apoptosis in ATL Cell Lines and CD30+ATL Cells
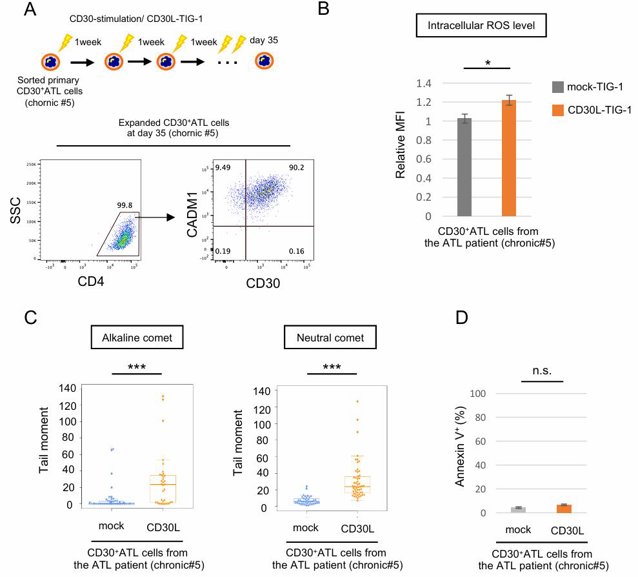
DNA double-strand breaks (DSBs) and oxidative stress due to an accumulation of reactive oxygen species (ROS) cause chromosomal instability, which is a hallmark of cancers. CD30, a receptor known to be critical for ATL progression, mediates survival signals. ATL is caused by HTLV-1 infection, and various genetic mutations and chromosome aberrations have been reported. Here, Nakashima et al. explored the potential role of CD30 signaling in chromosomal instability and ATL progression by investigating the effects of CD30 signaling on DSBs and ROS in ATL cells.
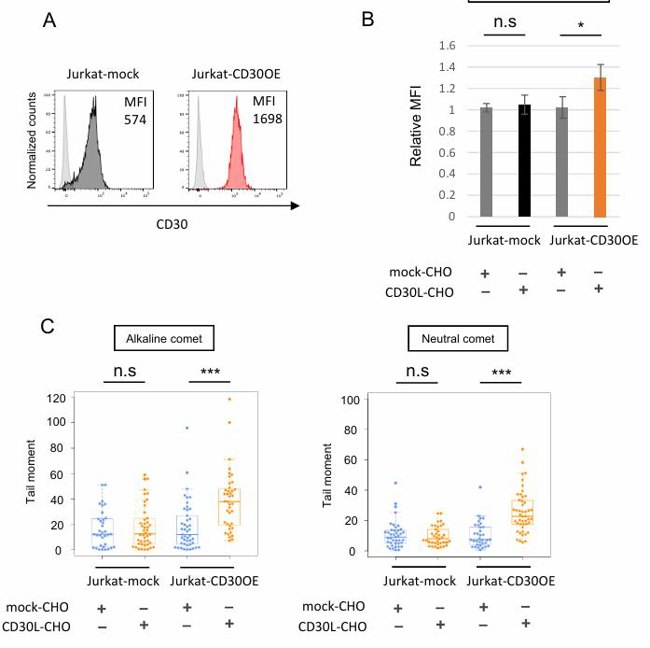
They used an intracellular ROS indicator (CellRox) to check if CD30 signaling increases intracellular ROS. Treatment with tBHP or TPA increased ROS in the ATL cell line HUT102, and the increased ROS by these treatments were suppressed by antioxidants NAC or GSH (Fig. 1A, B). Stimulation of CD30 by CD30L significantly increased ROS in CD30-expressing ATL cell lines (HUT102 and TL-Om1) and CD30+ATL cells from patients, while CD30-weak positive T-cell lines (Jurkat and CEM) and CD30− Ramos cells did not show elevated ROS (Fig. 1C, D; Fig. 2A, B). CD30 expression levels in primary CD30+ATL cells were higher than those in Jurkat and CEM cells and comparable to those in HUT102 cells but were lower than those in TL-Om1. Next, they overexpressed CD30 in Jurkat cells (Jurkat-CD30OE) and found that stimulation of CD30 by CD30L increased ROS in these cells (Fig. 3A, B). CD30 signaling did not induce apoptosis in ATL cell lines and CD30+ATL cells as assessed by annexin V binding (Fig. 1E; Fig. 2D). Thus, CD30 signaling elevates intracellular ROS in a CD30 expression level-dependent manner without inducing apoptosis.
BCL6 Maintains the Proliferation of HTLV-1-infected T Cells
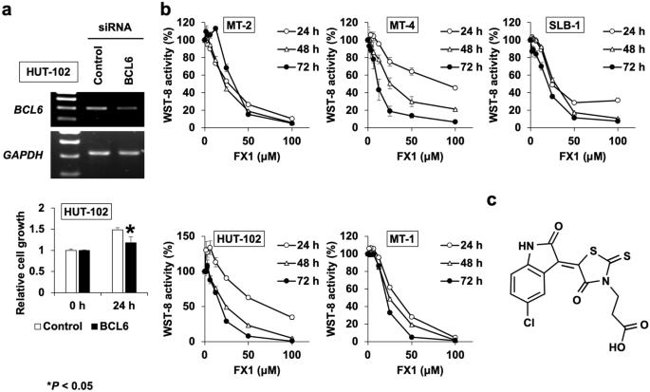
Adult T-cell leukemia (ATL) is an aggressive malignancy caused by HTLV-1 infection. The exact mechanisms of HTLV-1-induced leukemogenesis are unclear. The viral protein Tax activates survival pathways in ATL cells, but its loss is common in ATL cases. BCL6 is an oncogene responsible for the pathogenesis of B-cell malignancies, which represses several critical genes involved in the cell cycle checkpoint. In this study, Ishikawa et al. have examined the role of BCL6 in HTLV-1-induced leukemogenesis and the effect of its inhibitor FX1 on HTLV-1-infected T cells.
Since BCL6 has been known to facilitate the proliferation and survival of B cells, they sought to examine whether BCL6 also functions as a survival factor in HTLV-1-infected T cells. To address this question, HUT-102 cells were transfected with a specific siRNA for BCL6 and the cellular proliferation was analyzed at 24 h. The transfection efficiency was confirmed by the measurement of BCL6 levels in transfected HUT-102 cells by RT-PCR. As shown in Fig. 4a, BCL6 mRNA expression was reduced in comparison to that in the control siRNA-transfected cells, and the knockdown of BCL6 expression resulted in the attenuation of HUT-102 proliferation as indicated by the decrease in WST-8 activity.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells