- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The GPNT cell line was isolated from the rat brain endothelial cell line derived from the previously described rat brain endothelial cell line. Brain endothelial cells are an integral part of the blood-brain barrier (BBB). These cells primarily perform the function of exchange between blood and brain tissue. In addition, on the surface of brain endothelial cells, there is a high expression of a multidrug efflux pump called P-glycoprotein (P-gp). It actively pumps lipophilic drugs (for example, anticancer drugs) out of the cell, creating a protective barrier.
Thus, the GPNT cell line can be used as an in vitro model for studying drug transport and P-gp, including the penetration of anticancer drugs through the BBB. In addition, GPNT cells can be used to test the BBB permeability of drugs, which will be useful in the development of new drugs.
Effects of Hyperammonaemia on the Natriuretic Peptide System in Rat GPNT Brain Endothelial Cells
CNP (central nervous system natriuretic peptide) is a regulator of cGMP production, and is essential for neurogenesis and memory. Hyperammonaemia is associated with many neurological diseases and has been shown to impair astrocyte function, and cause inhibition of cGMP pathways in astrocytes. Regan et al. used rat C6 glioma cells and rat GPNT endothelial cells to explore the effects of hyperammonaemia on CNP-stimulated cGMP accumulation, cell proliferation, ROS production and release of extracellular vesicles, using both molecular and pharmacological approaches.
The blood-brain barrier comprises astrocytes, pericytes, and endothelial cells. To determine if hyperammonaemia similarly inhibits natriuretic peptide signaling in brain endothelial cells, they conducted pharmacological experiments in rat GPNT cells. Multiplex RT-qPCR detected Npr1 and Npr2 transcripts (Fig. 1A). ANP and CNP both increased cGMP accumulation (Fig. 1B), and CNP activated GC-B in a concentration-dependent manner (Fig. 1C; EC50 ~34 nM). In contrast to the C6 cells, 1 mM SNP did not increase cGMP (Fig. 1B). In contrast to C6 cells, proliferation of GPNT cells was not affected by NH4Cl in the absence or presence of CNP/SNP (Fig. 1D–F). Pre-treatment of cells with 10 mM NH4Cl (1 h or 24 h) significantly inhibited CNP-stimulated cGMP accumulation (to 54.5 ± 11.5%at 1 h and 58.0 ± 3.7% at 24 h; Fig. 1G and H). they have found that both similar and unique hyperammonaemia sensitivities are present in GPNT compared to C6 cells.
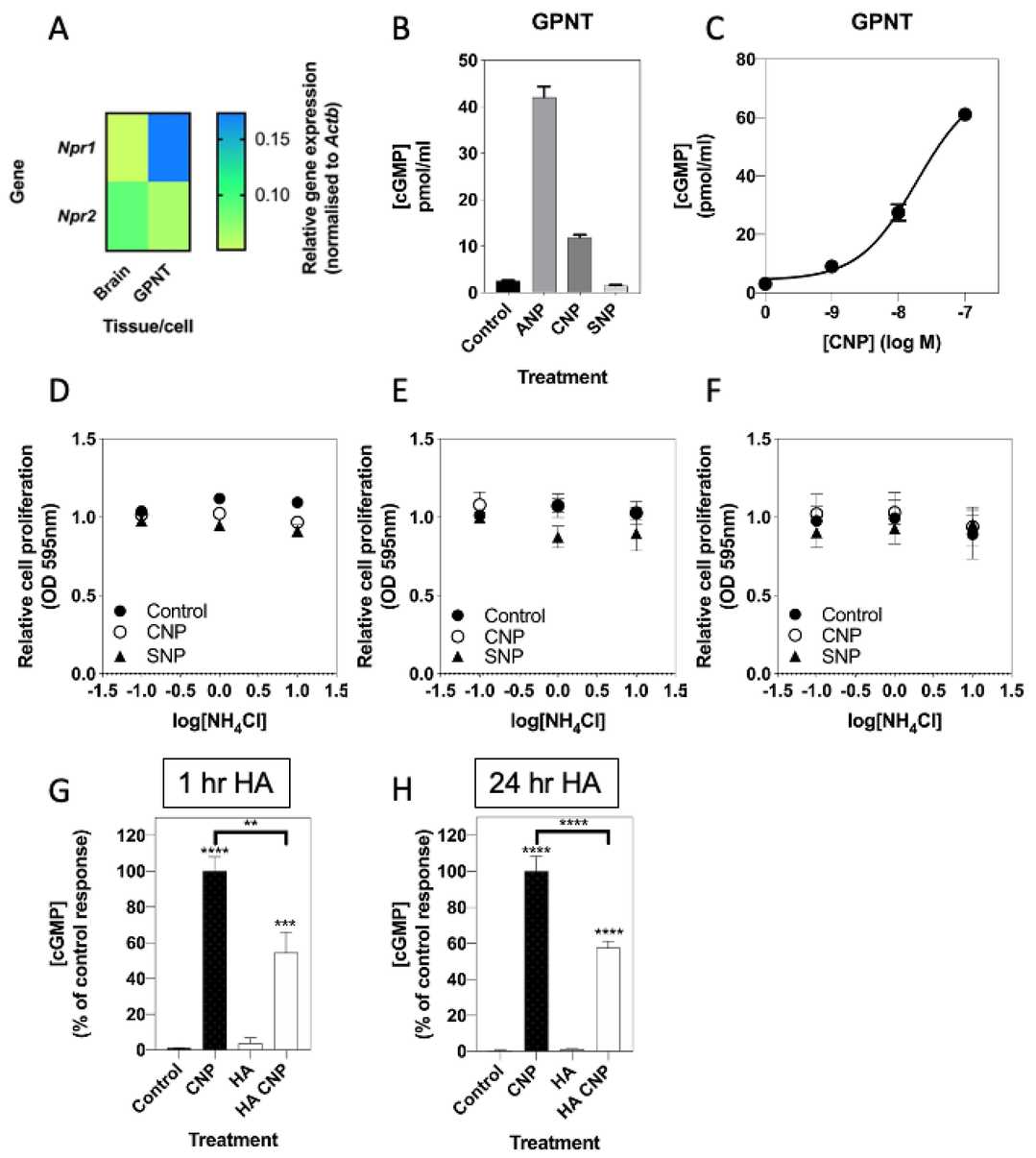 Fig. 1. Effects of hyperammonaemia on the natriuretic peptide system in rat GPNT brain endothelial cells (Regan JT, Mirczuk SM, et al., 2021).
Fig. 1. Effects of hyperammonaemia on the natriuretic peptide system in rat GPNT brain endothelial cells (Regan JT, Mirczuk SM, et al., 2021).
Endothelial PAR-1 Is Required for Lymphocyte Migration across Rat Brain Microvascular ECs
TEM of lymphocytes across BBB ECs involves ICAM-1-triggered signaling (e.g., JNK, AMPK, eNOS) and GPCR activation. PAR1, a protease-activated GPCR, regulates vascular function but its role in lymphocyte TEM is unknown. Dragoni et al. investigated whether PAR1 activation by lymphocyte-derived proteases is essential for TEM across BBB ECs and explores its interplay with established ICAM-1-mediated signaling pathways.
To study lymphocyte migration across brain microvascular endothelial cells (ECs), they used rat GPNT monolayers and the TEM-competent Th1 cell line PAS (Fig. 2A). Activated PAS cells were added to GPNT monolayers after 24 h of IL-2 stimulation. Broad-spectrum protease inhibitors (ATIII, leupeptin, benzamidine) reduced TEM by ≥60%, with benzamidine showing the strongest inhibition (<20% TEM), comparable to LFA1-ICAM-1 blockade (Fig. 2B, E) or GPCR inhibition. PAS cells resuspended in fresh medium (with/without IL-2) showed ~40% lower TEM, but migration recovered in 24 h preconditioned medium. Depleting serine proteases from this medium reduced TEM to <20%, mimicking benzamidine treatment (Fig. 2C). These results confirm that lymphocyte-derived serine proteases are essential for PAS TEM across GPNT BBB ECs.
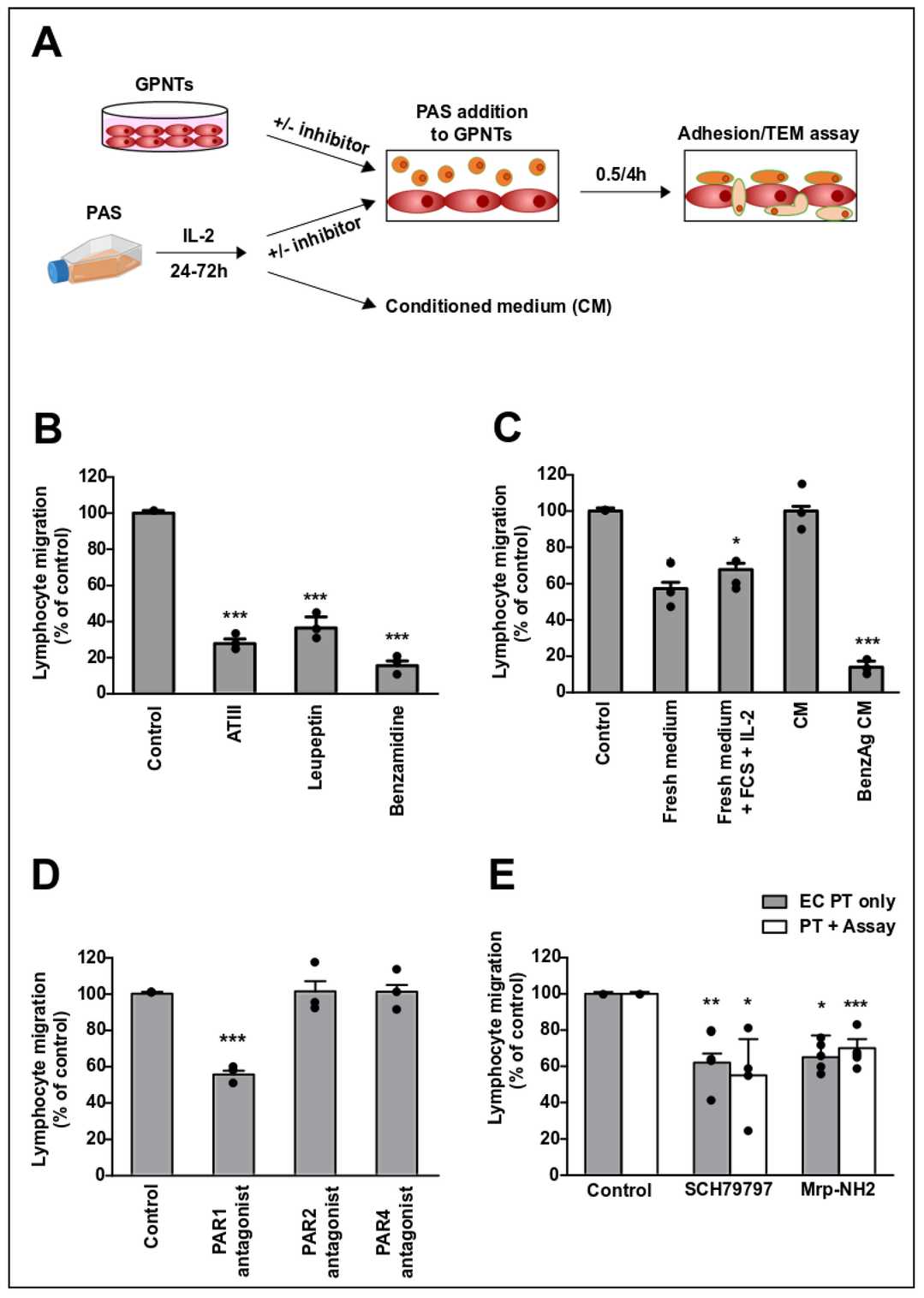 Fig. 2. A T cell-secreted protease activates endothelial PAR1 during TEM (Dragoni S, Papageorgiou A, et al., 2020).
Fig. 2. A T cell-secreted protease activates endothelial PAR1 during TEM (Dragoni S, Papageorgiou A, et al., 2020).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells