EPC
Cat.No.: CSC-C9050H
Species: Pimephales promelas (Fathead minnow)
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Epithelioma papulosum cyprini (EPC) cells are an immortalized fish epithelial cell line established from the skin papilloma of a young common carp (Cyprinus carpio). The common carp is an accessible and well-characterized aquatic species, making it an ideal candidate for establishing cell lines like EPC for research purposes. Naturally, EPC cells express epithelial-specific characteristics. They express tight junctions to model fish skin barrier function, and they express viral receptors to aid in the replication of aquatic viruses.
Their applications are prominent in aquatic science. Clinically, they are crucial for detecting aquatic viruses (e.g., spring viremia of carp virus, SVCV) and for developing vaccines (manufacturing inactivated viral antigens for use in fish vaccines, decreasing fish mortality and disease in aquaculture). Research-wise, EPC cells have been used to study fish epithelial physiology (e.g., elucidating the mechanisms that regulate the fish skin barrier), viral-host interactions (revealing the mechanisms of viral replication), and aquatic toxicology (assessing the effects of pollutants on fish epithelial health), among others.
Heterologous Overexpression of Human PML-IV in EPC Cells Results in Features of Premature Senescence
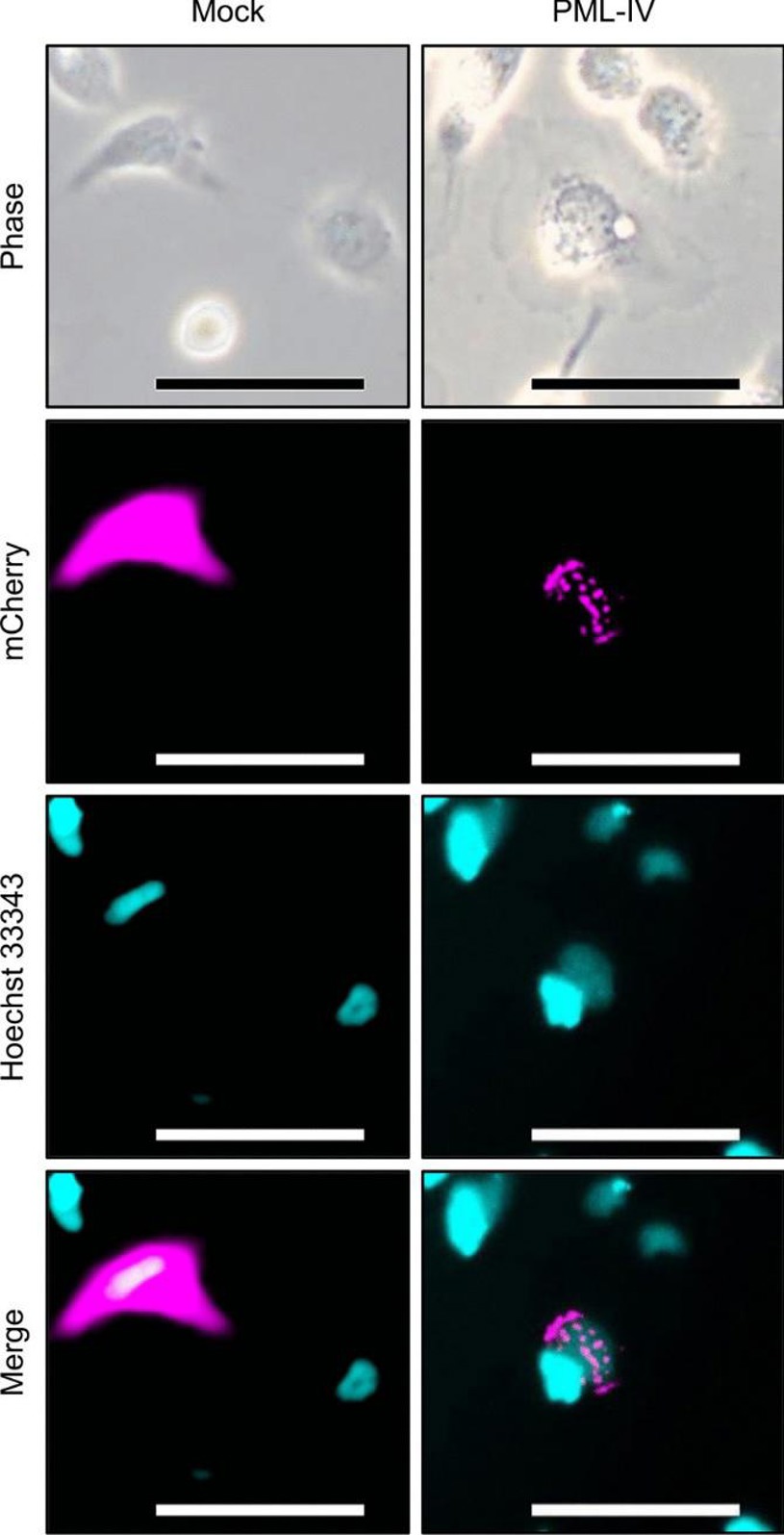
Fish cell lines show resistance to premature senescence, partly because fish genomes lack the p16INK4a/Arf locus. Other factors, such as the absence of PML NBs, may also be involved. In this study, human PML-IV was heterologously expressed in the Epithelioma papulosum cyprini cell line to induce the formation of PML NB-like structures. Futami et al. explored the link between the absence of PML NBs and resistance to senescence in fish cells.
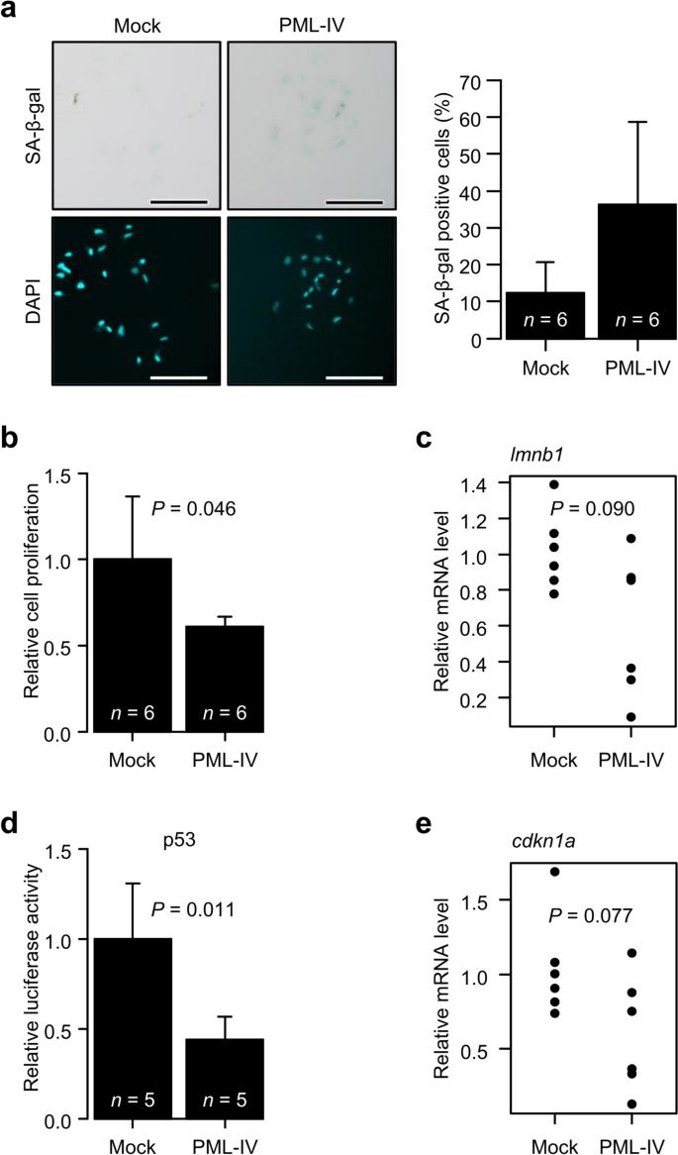
They transfected EPC cells with fluorescence-fused human PML-IV to induce PML NB formation and analyzed the cells using fluorescence microscopy (Fig. 1). Speckled PML NB-like structures were observed, but nuclear morphology remained unchanged. Phase contrast microscopy revealed that PML-IV-transfected cells exhibited the large, flattened morphology typical of senescent cells. They then assessed several senescence markers. SA-β-Gal activity increased (Fig. 2a), and an MTT assay confirmed senescence-like proliferation suppression (Fig. 2b). However, lmnb1 gene expression, which typically decreases in senescent cells, did not significantly change (Fig. 2c). Additionally, p53 activation and increased expression of its target gene p21 (cdkn1a) were not observed (Fig. 2d and 2e). These findings may explain the absence of nuclear morphological changes.
Exploring the Antiproliferative, Cytotoxic and Proapoptotic Properties of Virkon-S in Carp Epithelioma Papulosum Cells
Cyprinus carpio is a major farmed fish species in Turkey, but viral diseases such as Epithelioma papulosum cyprini (EPC) cause significant economic losses. Traditional disinfectants have potential genotoxic and cytotoxic effects, so non-toxic alternatives are needed. Virkon-S is a biocompatible disinfectant recommended by FAO, but its effects on EPC have not been studied.
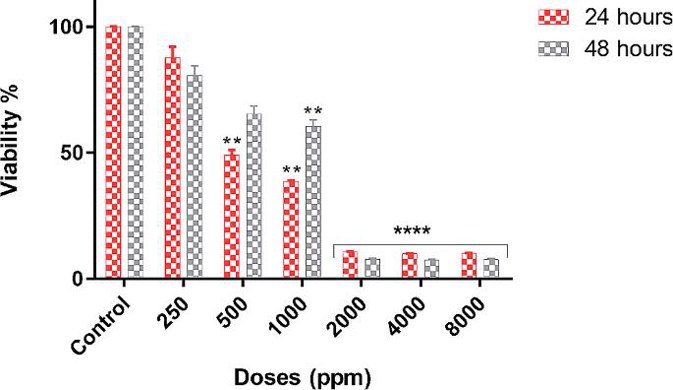
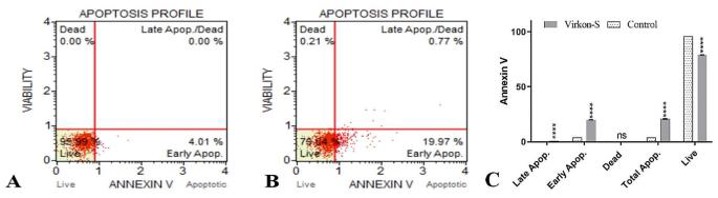
Izgördü et al. reported the cytotoxic, antiproliferative, and proapoptotic effects of Virkon-S in EPC cells by MTT assay, confocal and TEM microscopy, and Annexin-V and Caspase 3/7 analysis. IC50 values were 494.3 ppm (24 h) and 1198.3 ppm (48 h) in Virkon-S-treated EPC cells. The viability of EPC cells was significantly reduced as the increasing concentration and time of Virkon-S. These results showed that the cytotoxicity of Virkon-S is concentration- and time-dependent (Fig. 3). Annexin-V staining clearly demonstrated that Virkon-S induced apoptosis type cell death. The total percentage of apoptotic cells in Virkon-S-treated EPC cells was 20.74% while in control (Fig. 4A and B). Early apoptotic cells were found to be 4.01% in control and 19.97% in treated cells while late apoptotic cells were found to be 0.77% (Fig. 4B). The GraphPad Prism 8.0 program was used for statistical analysis (Fig. 4C).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells