BEN-MEN-1
Cat.No.: CSC-C1388
Species: Homo sapiens (Human)
Source: Brain
Morphology: adherent, epithelial cells growing as monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin-7 +, cytokeratin-8 -, cytokeratin-17 -, cytokeratin-18 +, cytokeratin-19 -, desmin -, endothel -, EpCAM -, GFAP -, neurofilament -, vimentin +
Viruses: PCR: EBV -, HBV -,
The BEN-MEN-1 cell line is human meningioma (WHO Grade I benign) cell line. The original tumor was derived from the falx cerebri of a 68-year-old female with meningioma after surgical resection. Falx cerebri is the dura mater in the brain near the ventricular system, which is the common site of meningioma and usually grows slowly with low malignant potential. BEN-MEN-1 cells exhibit typical meningeal epithelial cell morphology, characterized by whirlpool-like structures and the expression of epithelial membrane antigen (EMA), as well as desmosomes and interdigitating cell processes.
This cell line is often used for basic research and drug screening of meningiomas. Include cell cycle regulation, showing sensitivity to natural compounds such as cucurbitacin D and goyazensolide, which can induce G2/M phase cell cycle arrest and inhibit cell proliferation. In the research of apoptosis regulation, the overexpression of miR-34a-3p could reduce cell proliferation and promote apoptosis while inhibitors of miR-34a-3p could reduce the rate of apoptosis of cells, suggesting that miR-34a-3p might play an important role in the malignant transformation of meningiomas. In addition, BEN-MEN-1 cells have been used to model the effect of NF2 gene mutation on cell behavior and TAK-243 could significantly induce the apoptosis of BEN-MEN-1 cells. This cell line is also used to study the expression level of tumor-related genes. For example, when studying the effect of demethylating agents (Decitabine) on tumor-related genes, the expression level of DIO3 in BEN-MEN-1 cells could be used as a reference.
NF2-Deficient AC and MN Cells Secrete NRG1 Ligand that Activates the ERBB3 Receptor and Downstream Signaling
Meningiomas (MNs) are tumors arising from the meningeal layer, often unresponsive to chemotherapy, with approximately 50% showing loss of the NF2 tumor suppressor gene. Previous research indicated that NF2 loss activates mTORC1 and mTORC2 signaling. Recent omics studies identified activated EPH receptors and Src family kinases in NF2-deficient cells including human arachnoid cells (ACs) and an immortalized human MN line Ben-Men-1. Beauchamp et al. then tested whether NF2-deficient AC cells and Ben-Men-1 cells secrete the NRG1 ligand (Fig. 1B). Media were collected after 48 h in serum-free medium from both NF2(+) and NF2(−) AC-CRISPR cells, then concentrated by centrifugal filtration. Immunoblotting confirmed NRG1 secretion in the 48 h-concentrated medium (48 h-CM) from NF2(−) AC cells and Ben-Men-1 cells but not in 48 h-CM from NF2(+) ACs or 0 h-CM from NF2(−) ACs (Fig. 1C). qPCR analysis also showed that re-expression of NF2 in Ben-Men-1 cells strongly decreased NRG1 expression. In conditioned medium experiments, NF2(+) ACs were treated with medium from either NF2(+) or NF2(−) ACs. Cells treated with 48 h-CM from NF2(−) ACs showed elevated phosphorylation of ERBB3 Y1197 (pERBB3) and EPHA2 S897 (pEPHA2) and activated mTOR signaling (Fig. 1D). Stimulation of NF2-null ACs with exogenous NRG1 also activated pERBB3, pEPHA2, pS6K T389 (mTORC1), and pAkt S473 (mTORC2) (Fig. 1E). These findings indicate that loss of NF2 in AC or MN cells causes NRG1 secretion and ERBB3 autocrine activation, and that NRG1-ERBB3 signaling cross-talks with EPHA2 and activates downstream mTORC1/2 signaling.
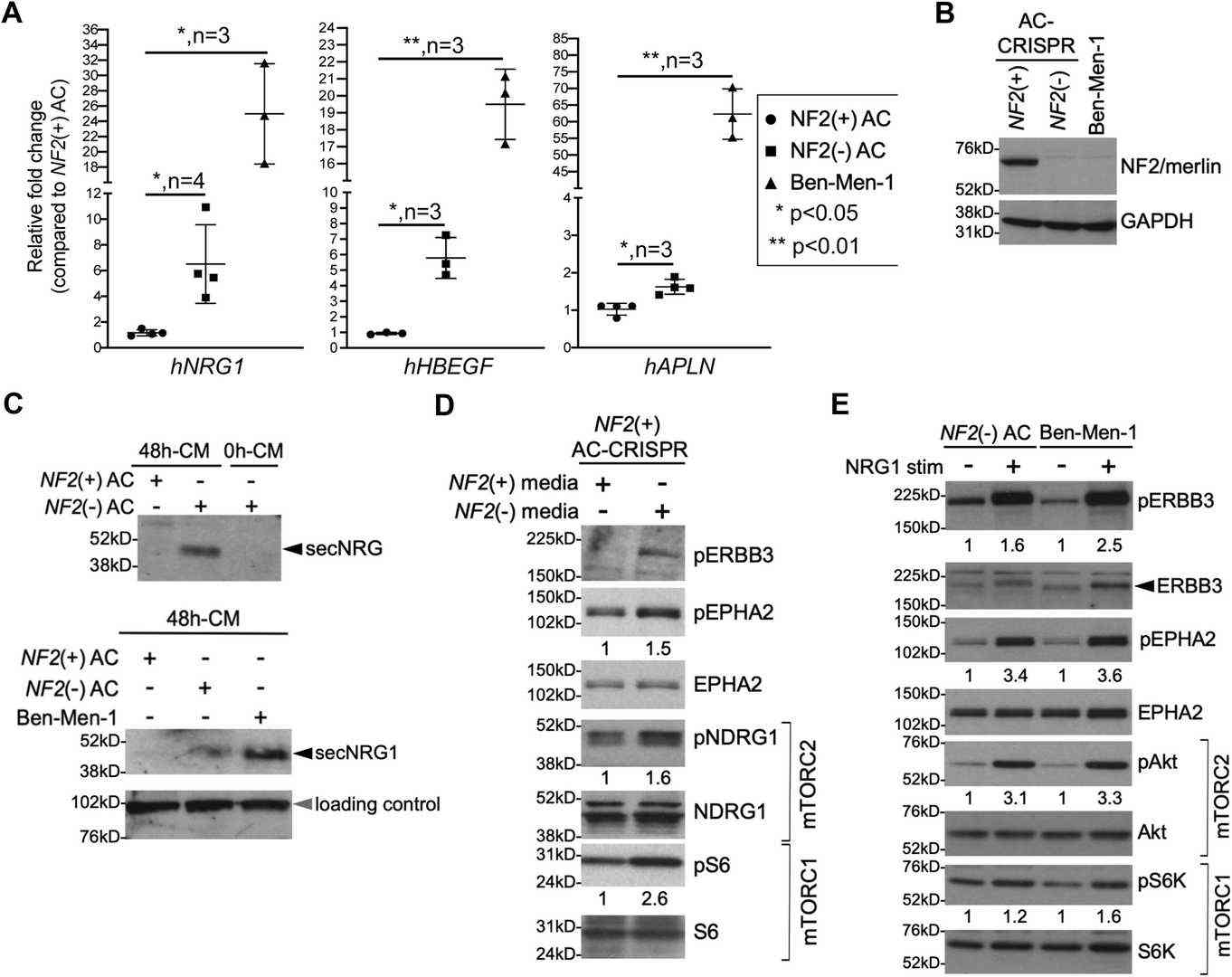 Fig. 1. NF2-null cells show increased NRG1 expression and secretion (Beauchamp R L, Erdin S, et al., 2021).
Fig. 1. NF2-null cells show increased NRG1 expression and secretion (Beauchamp R L, Erdin S, et al., 2021).
Expression of Sialyltransferases in Meningioma Cell Lines
Meningiomas are the most common non-malignant tumors of the intracranial region. They show anaerobic glycolysis, which leads to production of methylglyoxal (MGO) and glyoxal (GO), that can react with proteins to form AGEs. In this study, the effect of glycation on sialylation in meningioma was investigated using two cell lines (WHO grade I BEN-MEN-1 and WHO grade III IOMM-Lee) from patients with meningioma.
Figure 2A shows the expression of ST3GAL1–6 in BEN-MEN-1 and IOMM-Lee. ST3GAL1–ST3GAL3 and ST3GAL5–ST3GAL6 were detected in both BEN-MEN-1 and IOMM-Lee. ST3GAL2 showed higher band intensity in IOMM-Lee cells compared to BEN-MEN-1, while ST3GAL3 and ST3GAL5–6 showed higher intensity in BEN-MEN-1 cells. Figure 2B presents the expression of ST6GAL1–2. ST6GAL2 was not detected in IOMM-Lee cells, while ST6GAL1 showed no difference in band intensity between BEN-MEN-1 and IOMM-Lee cells. Figure 2C shows the expression of ST6GALNAC1–6. A weak band of ST6GALNAC2 was detected in BEN-MEN-1, while ST6GALNAC4–6 were detected in both BEN-MEN-1 and IOMM-Lee. ST6GALNAC5 and ST6GALNAC6 showed higher band intensity in IOMM-Lee cells. Figure 2D shows the expression of ST8SIA1–6. ST8SIA1–2 and ST8SIA5–6were detected in both BEN-MEN-1 and IOMM-Lee. ST8SIA2 and ST8SIA6 showed higher band intensity in BEN-MEN-1 cells while ST8SIA5 showed higher band intensity in IOMM-Lee cells.
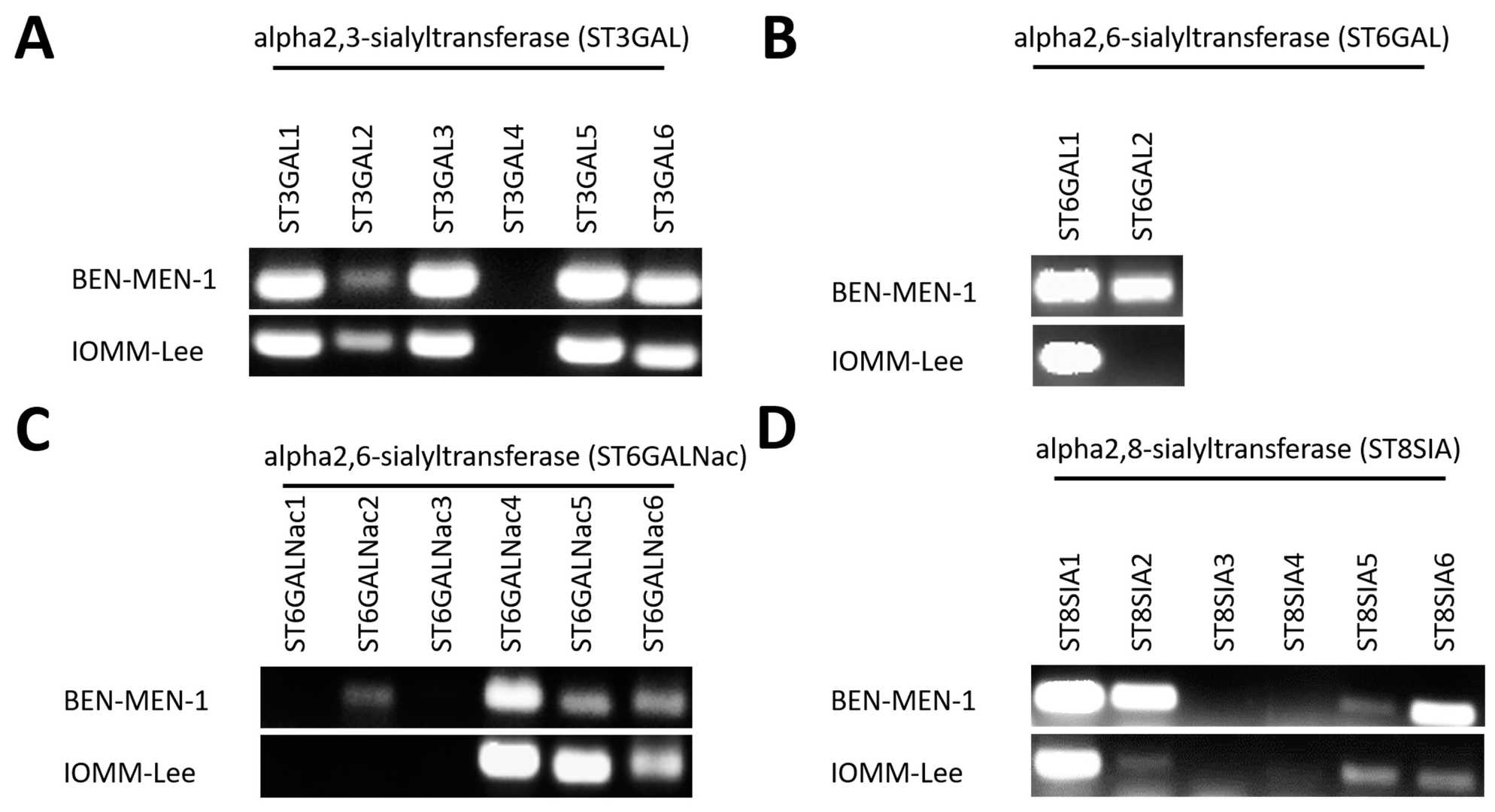 Fig. 2. Expression of 20 Sialyltransferases (agarose gel) in BEN-MEN-1 and IOMM-Lee (Selke P, Bork K, et al., 2021).
Fig. 2. Expression of 20 Sialyltransferases (agarose gel) in BEN-MEN-1 and IOMM-Lee (Selke P, Bork K, et al., 2021).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells