BE(2)-M17
Cat.No.: CSC-C9367J
Species: Homo sapiens (Human)
Source: Bone Marrow Metastasis
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
BE(2)-M17 are human neuroblastoma cells derived from bone marrow metastasis from a 2-year-old boy. These undifferentiated cells have a short spindle or irregular polygonal morphology and can grow as dense cultures of adherent growing cells. On induction with agents such as all-trans retinoic acid, BE(2)-M17 cells rapidly extend dendrite-like processes and assume a mature neuronal morphology and function. This process is also associated with a decrease in proliferation and cell cycle arrest in the G1/S phase. BE(2)-M17 cells display high proliferative potential and sensitivity to drugs and generally model the malignant properties of neuroblastoma. On a molecular level, these cells express baseline levels of the catecholamine neurotransmitter synthesizing and storage machinery, such as tyrosine hydroxylase and monoamine oxidase. In addition, neuronal differentiation is associated with a strong upregulation of neuronal marker expression including β-III tubulin and neurofilament protein, as well as functional aspects such as calcium dependent neurotransmitter release. BE(2)-M17 cells also express APP endogenously and can process APP to produce Aβ40/42. This line has been used extensively as an in vitro model for Parkinson's disease, Alzheimer's disease and neurotoxicology in general.
 Fig. 1. Photographs of BE(2)-M17 cell line (González V, Paz B, et al., 2017).
Fig. 1. Photographs of BE(2)-M17 cell line (González V, Paz B, et al., 2017).
The BE (2)-M17 Cell Line has a Better Dopaminergic Phenotype than the Traditionally Used for Parkinson´S Research SH-SY5Y, which is Mostly Serotonergic
SH-SY5Y, the most frequently used in vitro PD model, does not differentiate into a dopaminergic phenotype and mainly exhibits a serotonergic identity after differentiation. In this study, Caravjal-Oliveros et al. attempted to directly compare the dopaminergic and serotonergic marker profile of undifferentiated and differentiated SH-SY5Y and BE(2)-M17 neuroblastoma cells under the same conditions in order to assess their PD model potential.
Identical differentiation protocols with retinoic acid (RA) or staurosporine (Stau) were used to quantify dopaminergic and serotonergic markers in SH-SY5Y and BE(2)-M17 cells. Treatment with 10 μM RA or Stau (10 and 20 nM) resulted in a complete growth arrest of SH-SY5Y cells, whereas BE (2)-M17 cells were less susceptible. Morphological changes were observed after six days of RA treatment and were more evident at 12 days. Treatment with Stau for six days resembled the morphological state of 12 days of RA treatment. After 12 days of RA treatment or six days of Stau, neurites were thicker and the branch length decreased (Fig. 1). Tyrosine hydroxylase (TH), an enzyme essential for dopamine synthesis, was naturally expressed only in BE (2)-M17 cells. TH in BE (2)-M17 cells was significantly induced by RA after 12 days in culture (not in SH-SY5Y cells). Stau treatment further increased TH levels in BE (2)-M17 cells, which also decreased the induction time to six days. TH expression was only seen in SH-SY5Y cells when treated with 20 nM Stau. In all cases, the TH level was always significantly higher in BE (2)-M17 cells. The TH levels in SH-SY5Y cells after Stau treatment were, at most, at the same level of the initial TH levels of BE (2)-M17 cells (Fig. 2A and C).
![The cytotoxic effects of cisplatin, [Pt(η1-C2H4-OMe)(DMSO)(phen)]+(1), [Pt(η1-C2H4-OEt)(DMSO)(phen)]+ (2).](/upload/image/28-be2-m17-2.jpg) Fig. 1. Retinoic acid and staurosporine induce morphological changes consistent with neuronal differentiation in BE (2)-M17 and SH-SY5Y cells (Caravjal-Oliveros A, Uriostegui-Arcos M, et al., 2022).
Fig. 1. Retinoic acid and staurosporine induce morphological changes consistent with neuronal differentiation in BE (2)-M17 and SH-SY5Y cells (Caravjal-Oliveros A, Uriostegui-Arcos M, et al., 2022).
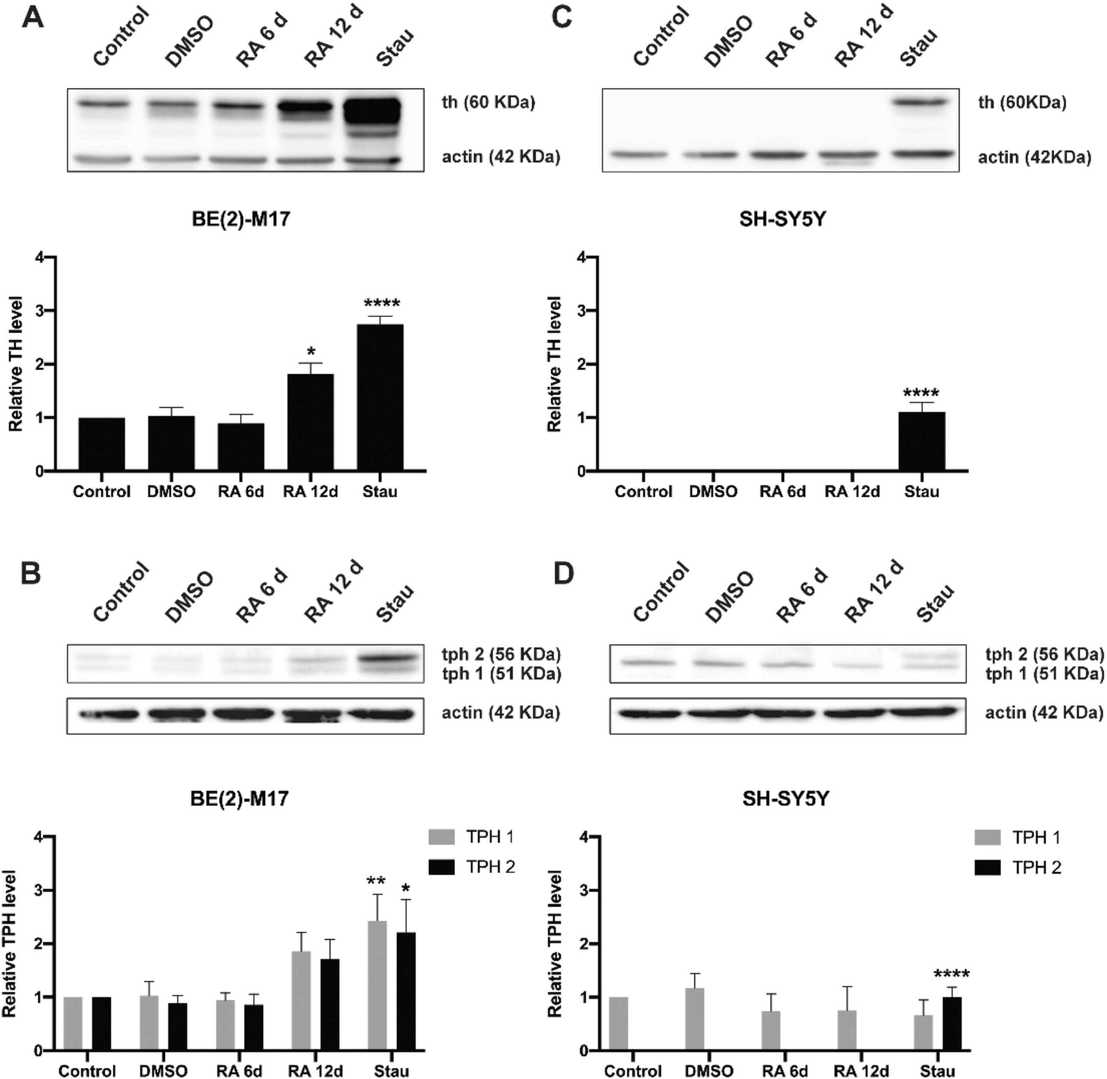 Fig. 2. Induction of tyrosine hydroxylase and tryptophan hydroxylase determined by western blot of untreated and treated BE (2)-M17 and SH-SY5Y cells (Caravjal-Oliveros A, Uriostegui-Arcos M, et al., 2022).
Fig. 2. Induction of tyrosine hydroxylase and tryptophan hydroxylase determined by western blot of untreated and treated BE (2)-M17 and SH-SY5Y cells (Caravjal-Oliveros A, Uriostegui-Arcos M, et al., 2022).
Protection of LM Compounds Against MPP⁺-Induced Neurotoxicity in BE(2)-M17 Cells
Parkinson's disease is driven by a positive feedback loop of microglial activation, oxidative stress, and neuron-derived neuroinflammation. The study focused on determining whether synthetic coumarin–chalcone hybrids LM-021 and LM-036 serve as dual NLRP1/NLRP3 inhibitors to protect human microglia HMC3 cells and neuroblastoma BE(2)-M17 cells from damage caused by MPP⁺ exposure.
Human BE(2)-M17 cells were exposed to different MPP⁺ concentrations (0–5 mM) for 20 h to study MPP⁺ cytotoxicity. MPP⁺ at 0.62 mM concentration was used for further experiments. BE(2)-M17 cells were treated with retinoic acid on day 1 to induce neuronal differentiation, and on day 5 after the removal of retinoic acid, the cells were pre-treated with LM compounds (1–10 μM) for 8 h and then MPP⁺ was added for 40 h (Fig. 3B). As shown in Figure 3C, LM-016, LM-021, and LM-036 at 10 μM concentration increased the cell viability (from 81 to 95–97%, p = 0.004–0.002) and decreased the LDH release in the cell culture medium (from 302 to 160–121%, p < 0.001). The observation of a decreased production of ROS (from 178 to 142–139%, p = 0.005–0.003) also supports the protective role of LM-016, LM-021, and LM-036 against MPP⁺-mediated neurotoxicity in BE(2)-M17 cells (Fig. 3D).
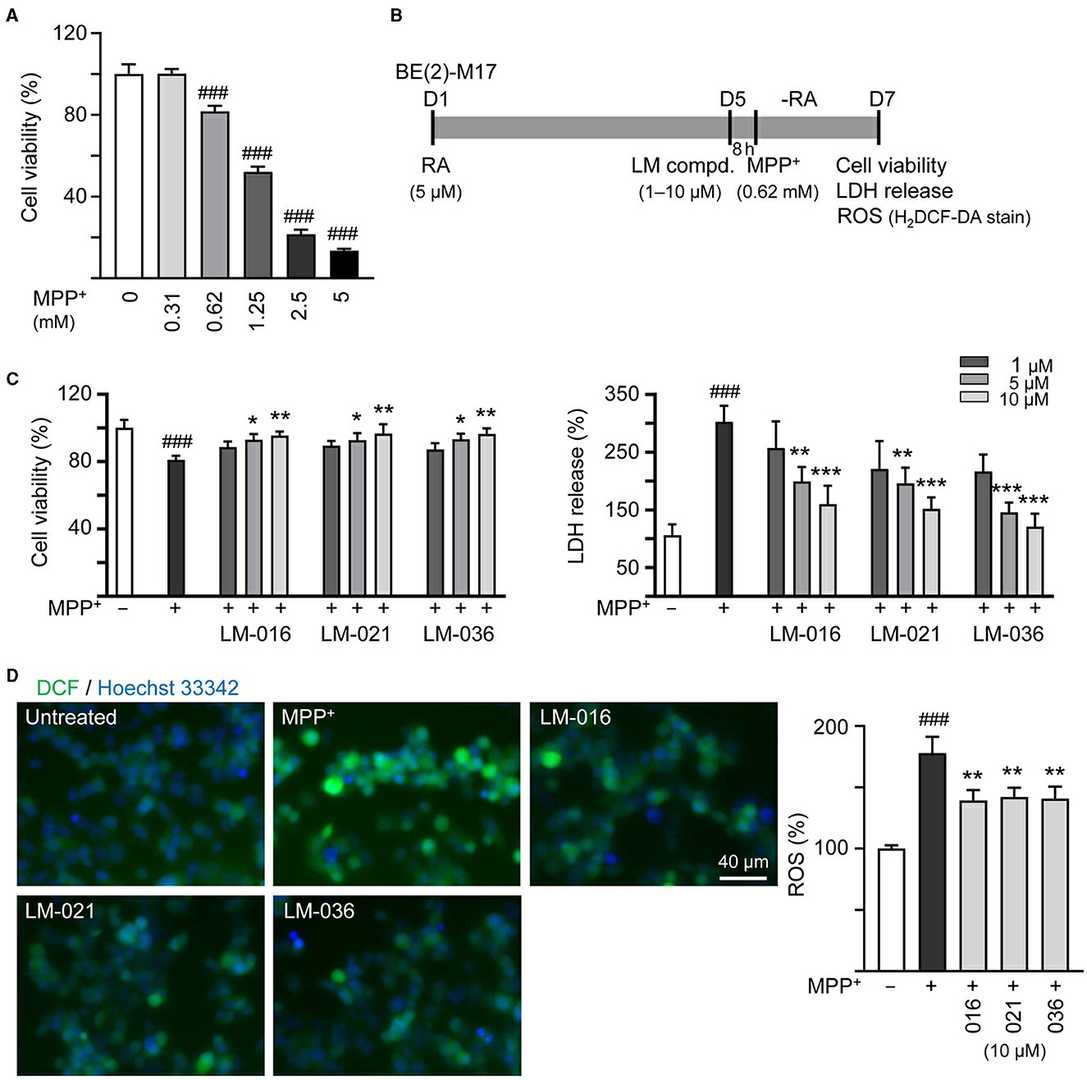 Fig. 3. Reduction of cytotoxicity and ROS of LM compounds in MPP⁺-treated BE(2)-M17 cells (Lin T, Chiu Y, et al., 2024).
Fig. 3. Reduction of cytotoxicity and ROS of LM compounds in MPP⁺-treated BE(2)-M17 cells (Lin T, Chiu Y, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells