P19.CL6
Cat.No.: CSC-C6314J
Species: Mus musculus (Mouse)
Morphology: other
Culture Properties: Adherent cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Store in liquid nitrogen.
The P19.CL6 cell line is a clone-derived cell line derived from mouse embryonal carcinoma cells (P19 embryonal carcinoma cells), isolated by Habara and Ohkubo in 1996. This cell line morphologically similar to the original P19 cells, which are round or flattened with typical stem cell characteristics. P19.CL6 cells display exponential growth during standard in vitro culture and transform into cardiomyocytes when exposed to specific conditions like 1% DMSO addition. The cell line shows mature heart muscle cell characteristics through α- and β-cardiac myosin heavy chain transcription and translation and displays calpain M emergence and contraction-related protein expression. P19.CL6 cells also show responsiveness to multiple signaling pathways including the BMP pathway which is essential for cardiac differentiation.
Due to their pluripotency and ease of differentiation, the P19.CL6 cell line is commonly used in research on cardiomyocyte differentiation. For instance, researchers apply this cell line in their investigations of how bone morphogenetic proteins (BMPs) control cardiac differentiation processes and for examining primary cilia functions during the initial stages of heart development. Moreover, researchers utilize P19.CL6 cells to examine cardiac repair mechanisms after myocardial infarction and to explore how intermittent hypoxia affects cardiomyocytes.
The Gene Expression Levels of Cd38, Ryr2, and Fkbp12.6 in Cardiomyocytes Were Decreased by IH
Sleep apnea syndrome (SAS) manifests as repeated oxygen desaturation and reoxygenation patterns known as intermittent hypoxia and poses risks for cardiovascular disease and insulin resistance/type 2 diabetes.
Takasawa et al. investigated the connections between IH stress and CVD. They treated rat H9c2 and mouse P19.CL6 cardiomyocytes with experimental IH or normoxia for 24 hours and examined mRNA expression levels of components in the Cd38-cyclic ADP-ribose (cADPR) signaling pathway. Using real-time reverse transcriptase-polymerase chain reaction (RT-PCR), they analyze mRNA levels of Cd38 (which encodes ADP-ribosyl cyclase/cyclic ADP-ribose [cADPR] hydrolase, EC 3.2.2.6), Ryr2 and Fkbp12.6 (a cADPR receptor [Fkbp1b]) in mouse P19.CL6 and rat H9c2 cells. IH led to significant reductions in mRNA levels of Cd38, Ryr2, and Fkbp12.6 within mouse P19.CL6 cardiomyocytes and rat H9c2 cardiomyocytes as demonstrated by Figure 1 and Figure 2. The researchers also evaluated the protein levels of Cd38, Ryr2, and Fkbp12.6 in H9c2 cells through Western blot assays and discovered significant reductions caused by IH with statistical significance values of p = 0.0205, p = 0.0241, and p = 0.0078, respectively (Fig. 3).
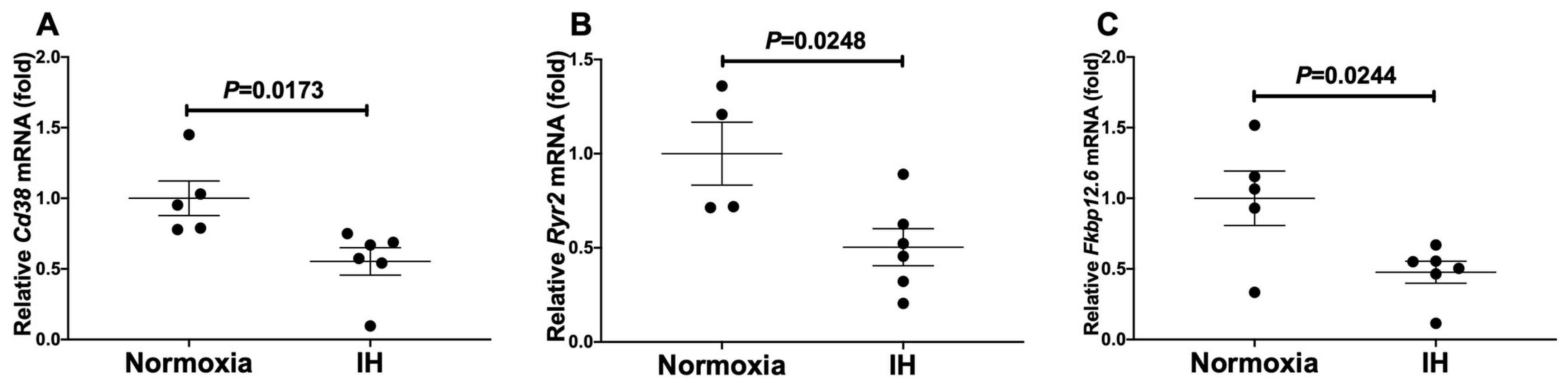 Fig. 1. The mRNA levels of mouse (A) Cd38, (B) Ryr2, and (C) Fkbp12.6 in mouse P19.CL6 cardiomyocytes (Takasawa S, Makino M, et al., 2022).
Fig. 1. The mRNA levels of mouse (A) Cd38, (B) Ryr2, and (C) Fkbp12.6 in mouse P19.CL6 cardiomyocytes (Takasawa S, Makino M, et al., 2022).
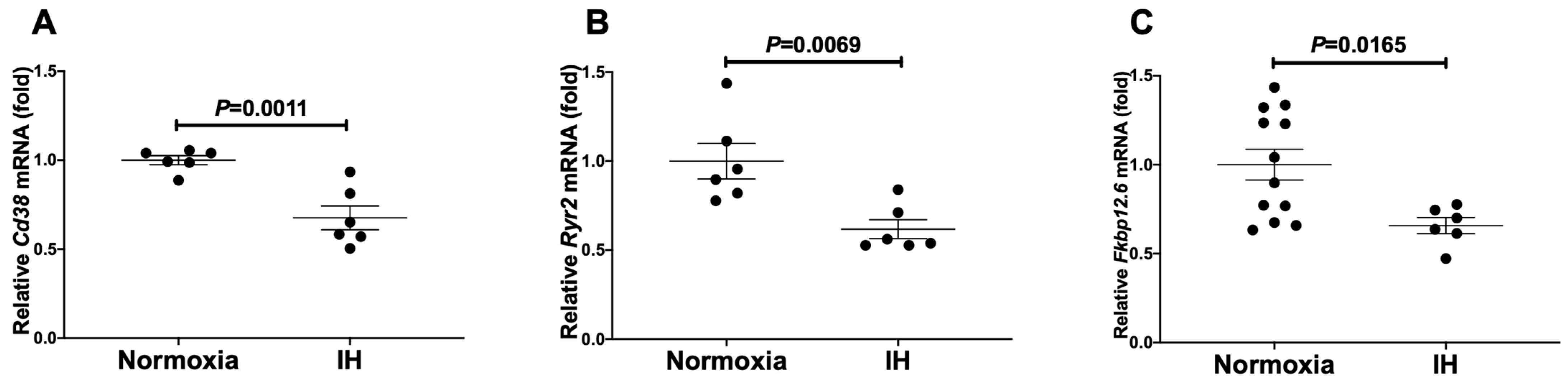 Fig. 2. The mRNA levels of mouse (A) Cd38, (B) Ryr2, and (C) Fkbp12.6 in rat H9c2 cardiomyocytes (Takasawa S, Makino M, et al., 2022).
Fig. 2. The mRNA levels of mouse (A) Cd38, (B) Ryr2, and (C) Fkbp12.6 in rat H9c2 cardiomyocytes (Takasawa S, Makino M, et al., 2022).
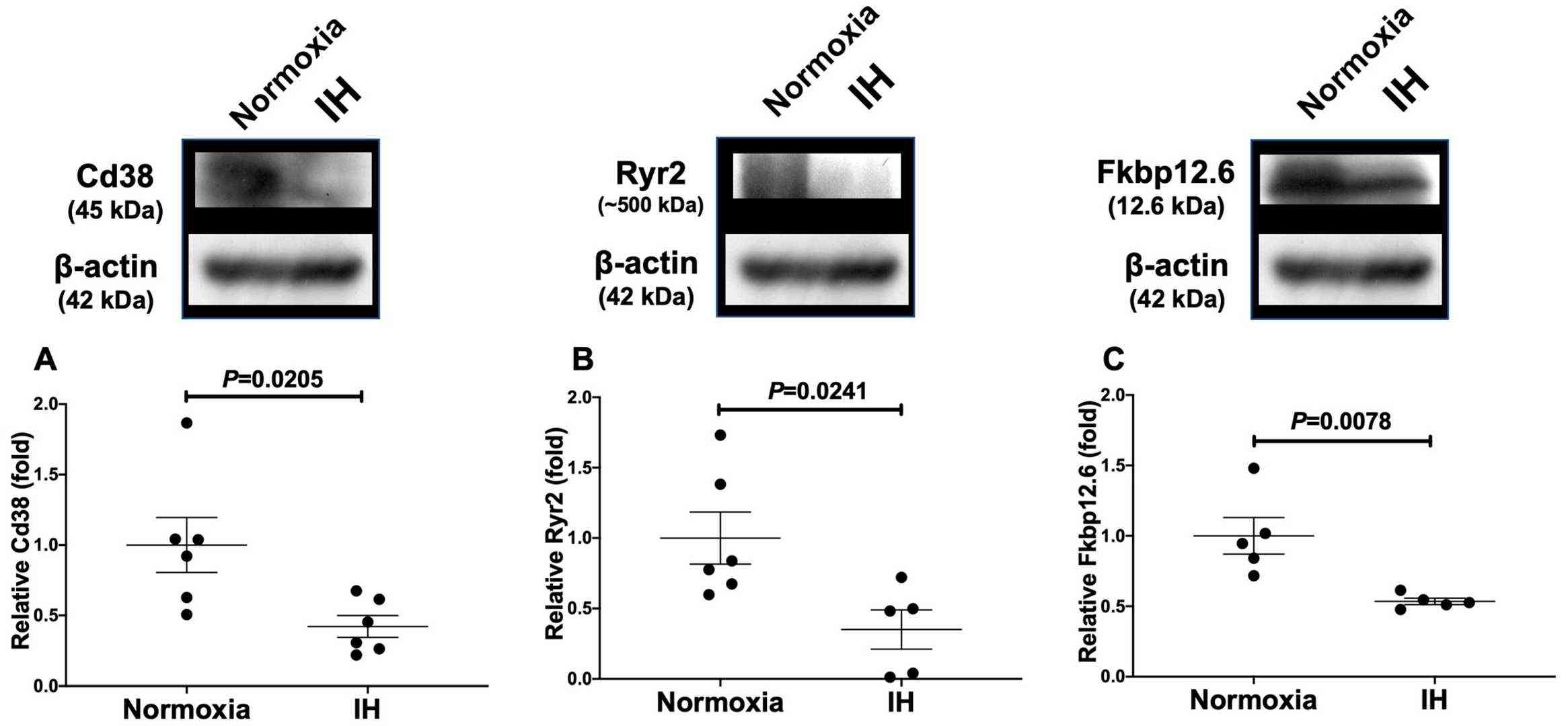 Fig. 3. Relative protein expression levels of (A) Cd38, (B) Ryr2, and (C) Fkbp in rat H9c2 cardiomyocytes subjected to IH (Takasawa S, Makino M, et al., 2022).
Fig. 3. Relative protein expression levels of (A) Cd38, (B) Ryr2, and (C) Fkbp in rat H9c2 cardiomyocytes subjected to IH (Takasawa S, Makino M, et al., 2022).
Gene Expressions of Reg IV and Hgf Were Increased by IH in Cardiomyocytes
The study focuses on sleep apnea syndrome (SAS), which causes intermittent hypoxia (IH) and consequently is a significant risk factor for cardiovascular diseases (CVD) and metabolic disorders like insulin resistance and Type 2 diabetes. SAS affects oxygen levels during sleep, resulting in numerous health complications, including CVD. Takasawa et al. aims to explore the mechanisms underlying how IH influences cardiomyocyte gene expression.
They treated rat H9c2 cardiomyocytes and mouse P19.CL6 cells under normoxia, intermittent hypoxia (IH), or sustained hypoxia (SH) for 24 hours. They measured the mRNA levels related to inflammation, growth, and function in these cells, including genes like Il-6, Tgfβ1, Ccl2, Tnfα, Vegf-A, Cd38, Reg genes, and Hgf using real-time RT-PCR. In rat H9c2 cells, Tgfβ1, Ccl2, Tnfα, Flt-1, Reg IV, and Hgf increased significantly with IH, as shown in Figure 4. In contrast, only Reg IV and Hgf mRNAs were significantly increased by IH in mouse P19.CL6 cells (Fig. 4). ELISA revealed that IH significantly raised Reg IV and Hgf protein levels in P19.CL6 cell media (Reg IV: 30.16 pg/mL vs. 91.83 pg/mL, p = 0.0025, and Hgf: 101.9 pg/mL vs. 106.3 pg/mL, p = 0.0046) (Fig. 5).
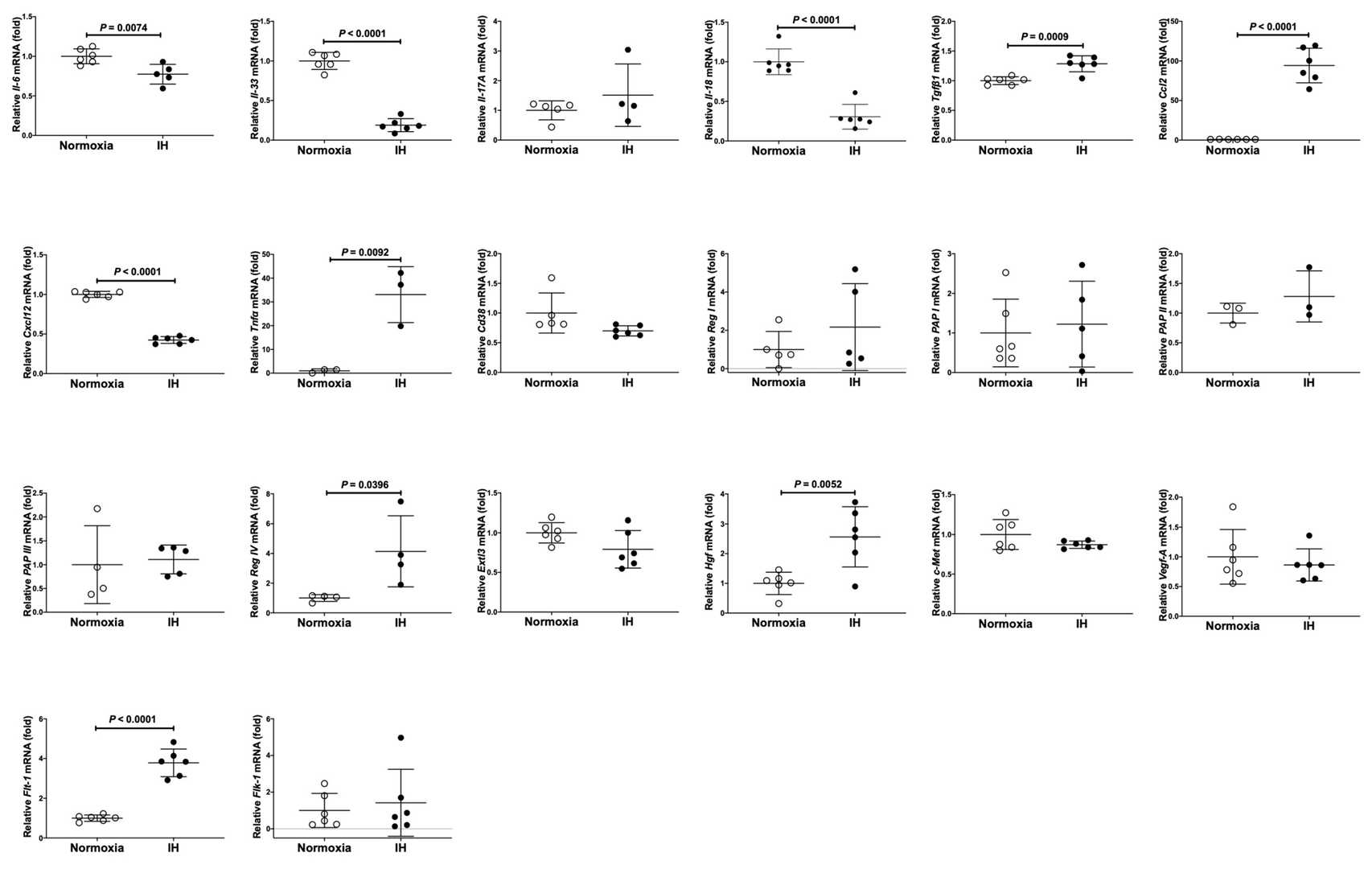 Fig. 4. The mRNA levels of rat Il-6, Il-33, Il-17A, Il18, Tgfβ1, Cxcl12, Tnfα, Ccl2, Vegf-A, Flt-1, Flk-1, Cd38, Reg I, PAP I, PAP II, PAP III, Reg IV, Extl3, Hgf, and c-Met in rat H9c2 cardiomyocytes (Takasawa S, Itaya-Hironaka A, et al., 2022).
Fig. 4. The mRNA levels of rat Il-6, Il-33, Il-17A, Il18, Tgfβ1, Cxcl12, Tnfα, Ccl2, Vegf-A, Flt-1, Flk-1, Cd38, Reg I, PAP I, PAP II, PAP III, Reg IV, Extl3, Hgf, and c-Met in rat H9c2 cardiomyocytes (Takasawa S, Itaya-Hironaka A, et al., 2022).
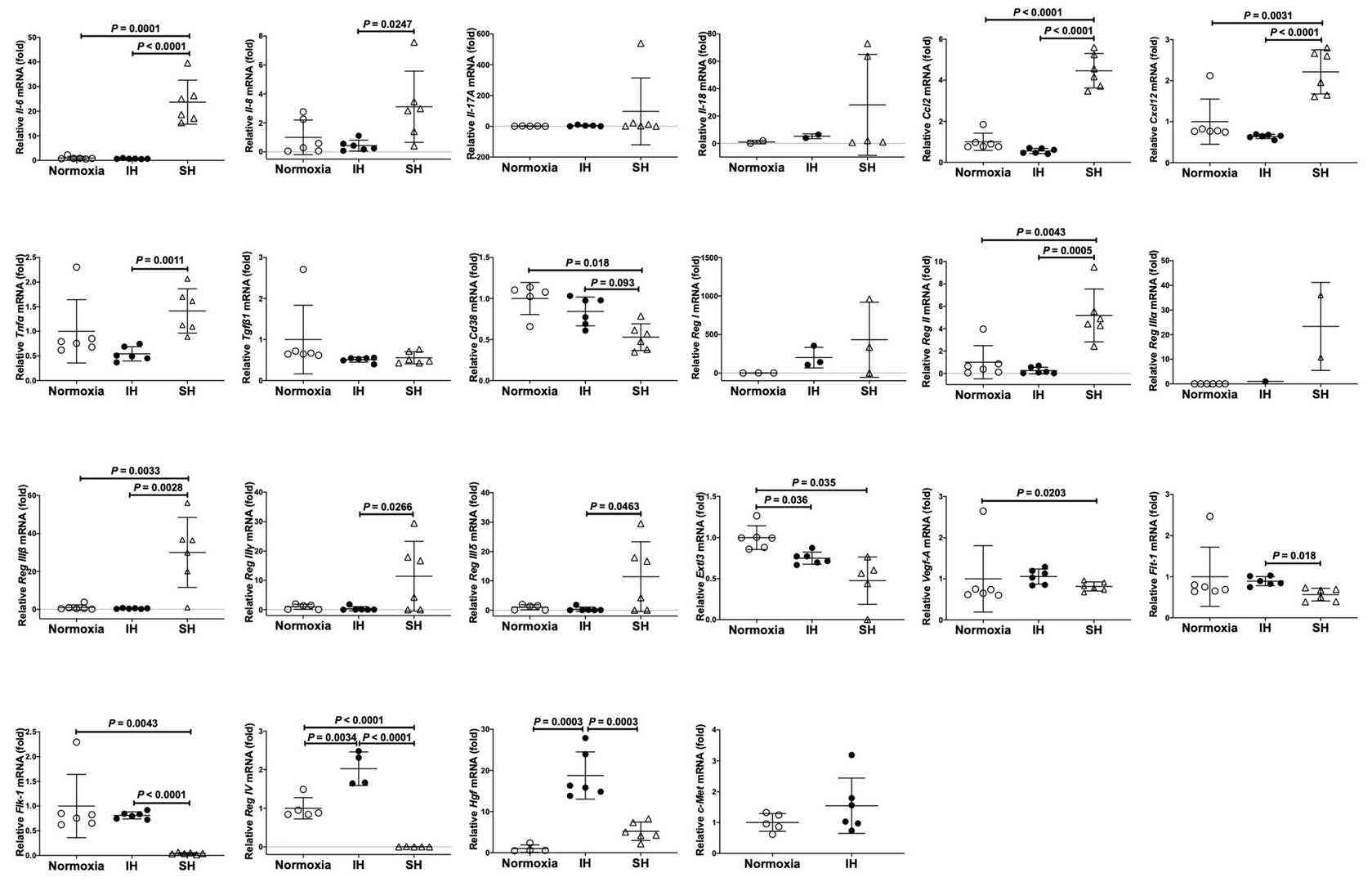 Fig. 5. The mRNA levels of mouse Il-6, Il-8, Il-17A, Il-18, Tgfβ1, Ccl2, Cxcl12, Tnfα, Vegf-A, Flt-1, Flk-1, Cd38, Reg I, Reg II, Reg IIIα, Reg IIIβ, Reg IIIγ, Reg IIIδ, Reg IV, Extl3, Hgf, and c-Met in mouse P19.CL6 cardiomyocytes (Takasawa S, Itaya-Hironaka A, et al., 2022).
Fig. 5. The mRNA levels of mouse Il-6, Il-8, Il-17A, Il-18, Tgfβ1, Ccl2, Cxcl12, Tnfα, Vegf-A, Flt-1, Flk-1, Cd38, Reg I, Reg II, Reg IIIα, Reg IIIβ, Reg IIIγ, Reg IIIδ, Reg IV, Extl3, Hgf, and c-Met in mouse P19.CL6 cardiomyocytes (Takasawa S, Itaya-Hironaka A, et al., 2022).
 Fig. 6. Concentrations of (A) Reg IV and (B) Hgf in a mouse P19.CL6 cardiomyocyte culture medium (Takasawa S, Itaya-Hironaka A, et al., 2022).
Fig. 6. Concentrations of (A) Reg IV and (B) Hgf in a mouse P19.CL6 cardiomyocyte culture medium (Takasawa S, Itaya-Hironaka A, et al., 2022).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells