YAPC
Cat.No.: CSC-C0430
Species: Homo sapiens (Human)
Source: Ascites
Morphology: cobblestone-like adherent cells growing in monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin +, cytokeratin-7 +, cytokeratin-8 +, cytokeratin-17 (+), cytokeratin-18 +, cytokeratin-19 +, desmin -, endothel -, EpCAM +, GFAP -, neurofilament
The YAPC cell line was developed in 1993 by the National Center for Global Health and Medicine, Japan from the ascites of a 43-year-old male patient with untreated advanced pancreatic cancer. YAPC cells are adherent and autocrine IL-1α-dependent for proliferation. Immunophenotypically, YAPC is positive for several adhesion molecules including cytokeratin, EpCAM, and vimentin and exhibits typical epithelial morphology. Genotypically, YAPC is near-triploid (8% polyploid) and has several chromosomal deletions and translocations associated with pancreatic cancer. Because of its natural resistance to cisplatin (24 h IC50 > 100 µM), YAPC is commonly used in drug development and research as an in vitro model for drug resistance, drug screening and drug combination research. YAPC has been used to screen novel platinum/organometallic compounds, 3D tumor spheroid invasion, KRAS-targeted therapy, Wnt/β-catenin signaling and the mitochondrial apoptosis pathway, among many others.
Cytotoxicity of Cisplatin in Pancreatic Tumor Lines
Pancreatic ductal adenocarcinoma (PDAC) is highly lethal and chemoresistant; gemcitabine frequently fails and cisplatin shows variable efficacy. Muscella et al. aimed to clarify why four genotypically distinct PDAC (BxPC-3, Mia-Paca-2, PANC-1, and YAPC) lines respond differently to cisplatin, with the goal of identifying molecular determinants of resistance. They treated the four lines with 0.1–200 µM cisplatin and measured viability by SRB (validated by cell counts). Figure 1 and Supplementary Table S1 show a dose- and time-dependent drop in survival: BxPC-3 and MIA PaCa-2 were most sensitive (IC50 = 5.96 ± 2.32 µM and 7.36 ± 3.11 µM at 48 h), whereas YAPC and PANC-1 were resistant (IC50 = 56.7 ± 9.52 µM and 100 ± 7.68 µM). Subsequent work focused on the contrasting pair BxPC-3 and YAPC.
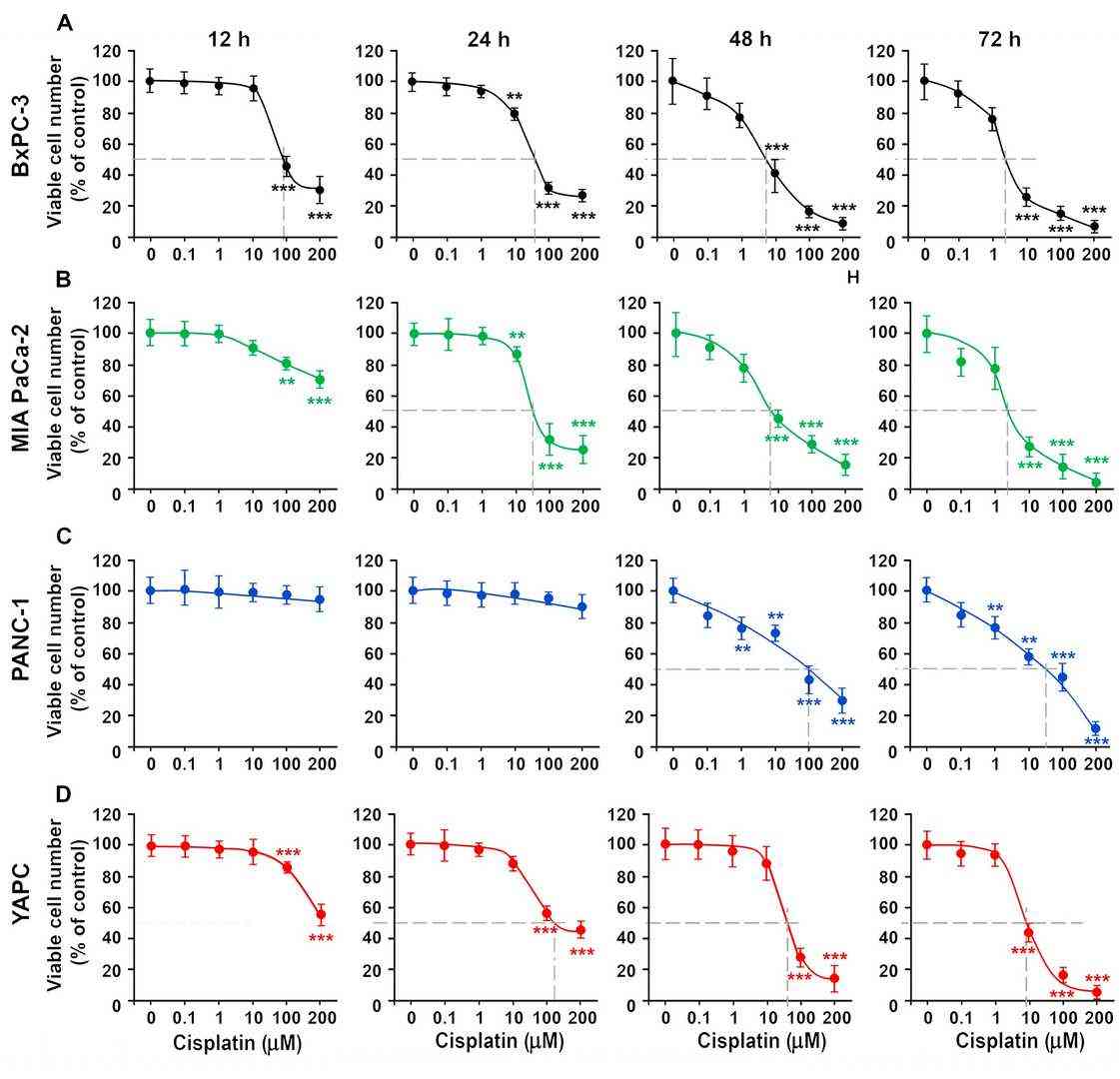 Fig. 1. Cytotoxic effects of cisplatin on pancreatic tumor lines. BxPC-3 (A), Mia Paca-2 (B), PANC-1 (C) and YAPC (D) cells were treated with different concentration of cisplatin (Muscella A, Cossa LG, et al., 2024).
Fig. 1. Cytotoxic effects of cisplatin on pancreatic tumor lines. BxPC-3 (A), Mia Paca-2 (B), PANC-1 (C) and YAPC (D) cells were treated with different concentration of cisplatin (Muscella A, Cossa LG, et al., 2024).
[Pt(η1-C2H4-OMe)(DMSO)(phen)]+ (1) and [Pt(η1-C2H4-OEt)(DMSO)(phen)]+ (2) Inhibition of PDAC Cell Growth
PDAC chemoresistance limits cisplatin efficacy. Three human PDAC lines (Mia PaCa-2, PANC-1, YAPC) were exposed to complexes 1 and 2 or cisplatin; SRB, apoptosis, cell-cycle, mitochondrial membrane potential, and 3D-spheroid migration assays were employed. The goal was to determine if 1 and 2 display superior antiproliferative and antimetastatic effects versus cisplatin. SRB assay on Mia PaCa-2, PANC-1 and YAPC showed both [Pt(η1-C2H4-OR)(DMSO)(phen)]+ complexes (R = Me, 1; Et, 2) killed cells in a concentration-dependent manner (0–100 µM, Fig. 2). After 24 h, 1 and 2 were markedly more potent than cisplatin (IC50 9.15–31.6 µM vs >100 µM for PANC-1; Fig. 3). Only Mia PaCa-2 displayed lower IC50 for cisplatin (3.76 ± 1.11 µM) at 48 h (Fig. 2b, Fig. 3). Chain length (Me vs Et) had minimal impact.
![The cytotoxic effects of cisplatin, [Pt(η1-C2H4-OMe)(DMSO)(phen)]+(1), [Pt(η1-C2H4-OEt)(DMSO)(phen)]+ (2).](/upload/image/27-yapc-2.jpg) Fig. 2. Cytotoxic efects of cisplatin, [Pt(η1-C2H4-OMe)(DMSO)(phen)]+(1), [Pt(η1-C2H4-OEt)(DMSO)(phen)]+ (2) (Stefàno E, Cossa L G, et al., 2023).
Fig. 2. Cytotoxic efects of cisplatin, [Pt(η1-C2H4-OMe)(DMSO)(phen)]+(1), [Pt(η1-C2H4-OEt)(DMSO)(phen)]+ (2) (Stefàno E, Cossa L G, et al., 2023).
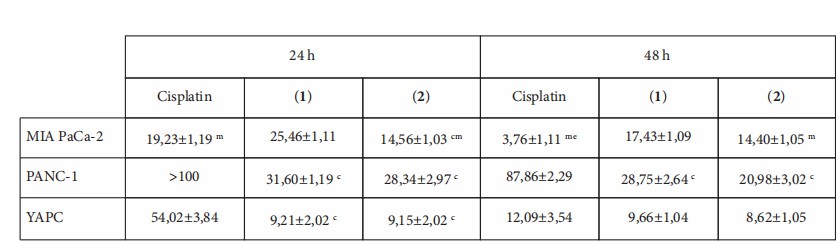 Fig. 3. IC50 values were calculated and presented as means ± standard deviation. Letters indicate IC50 values that are significantly lower (p < 0.05) than those of cisplatin (c), 1 (m), and 2 (e) at the same time point (Stefàno E, Cossa L G, et al., 2023).
Fig. 3. IC50 values were calculated and presented as means ± standard deviation. Letters indicate IC50 values that are significantly lower (p < 0.05) than those of cisplatin (c), 1 (m), and 2 (e) at the same time point (Stefàno E, Cossa L G, et al., 2023).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells