SVEC4-10
Cat.No.: CSC-C9132W
Species: Mus musculus (Mouse)
Source: Lymph Node Metastasis
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
SVEC4-10 represents a SV40-immortalized endothelial cell line originates from mouse axillary lymph nodes which serves as lymphatic endothelium. The SV40 virus (Simian Virus 40) provides immortality to this cell line while it continues to show normal lymphatic endothelial cell morphology and function. The SVEC4-10 cell line expresses endothelial markers like CD31 (PECAM-1), vWF (von Willebrand factor), and VE-cadherin while forming lumen-like structures that replicate angiogenic processes.
Researchers use the SVEC4-10 cell line for studies in lymphangiogenesis and angiogenesis as well as immune regulation and toxicity research:
Lymphangiogenesis and Metastasis: Researchers use SVEC4-10 cells to explore lymphangiogenesis mechanisms which play a vital role in tumor progression through cancer cell metastasis. SVEC4-10 cells facilitate the creation of lymphatic vessels while also supporting cancer cell metastasis through the secretion of VEGF and additional factors.
Immunological Research: The antigen presentation abilities of SVEC4-10 cells can be improved through the application of external MHC molecules or stimulatory factors like IFN-γ despite their inherent limited performance. Researchers utilize them to study immune system regulation along with cellular immune reactions.
Drug Screening and Toxicity Evaluation: SVEC4-10 cells are frequently used to evaluate chemical toxicities on endothelial cells because they demonstrate high sensitivity to oxidative stress and chemical exposure.
Angiogenesis and Regenerative Medicine: In angiogenesis and lymphangiogenesis simulations SVEC4-10 cells function as models which permit the examination of angiogenic factor expression and regulatory mechanisms.
3-BrFlu Induces both MAPK Phosphorylation and Intracellular ROS Accumulation in SVEC4-10 Endothelial Cells
3-Bromofluoranthene (3-BrFlu), a PAH derivative, is implicated in endothelial damage through pro-inflammatory pathways. Li's team utilizes SVEC4-10 cell lines and zebrafish models to investigate 3-BrFlu's impact on vascular endothelial dysfunction, specifically examining the role of the MAPK-mediated NF-κB pro-inflammatory pathway and intracellular ROS generation.
MAPK phosphorylation of p38 MAPK, ERK and JNK functions as a primary upstream regulator for NFκB p65. Immunoblotting analysis revealed that 3-BrFlu triggers MAPK phosphorylation starting at 3 μM concentration and this effect becomes significant at that level (Fig. 1). Phosphorylation levels of p38 MAPK rose from 1.86 to 2.69 times, ERK levels increased from 1.31 to 1.94 times, and JNK levels elevated from 1.77 to 2.73 times when treated with 3–100 μM 3-BrFlu. Intracellular ROS which are essential for maintaining vascular barrier functionality demonstrated concentration-dependent accumulation beginning at 3 μM as detected by a DCFH-DA fluorescent probe (Fig. 2). The fluorescence intensity of ROS demonstrated an increase from 1.04 × 104 to 1.83 × 104–3.56 × 104 when exposed to concentrations ranging from 10 to 100 μM of 3-BrFlu. The experimental results show that 3-BrFlu causes both MAPK phosphorylation and intracellular ROS accumulation in SVEC4-10 endothelial cells.
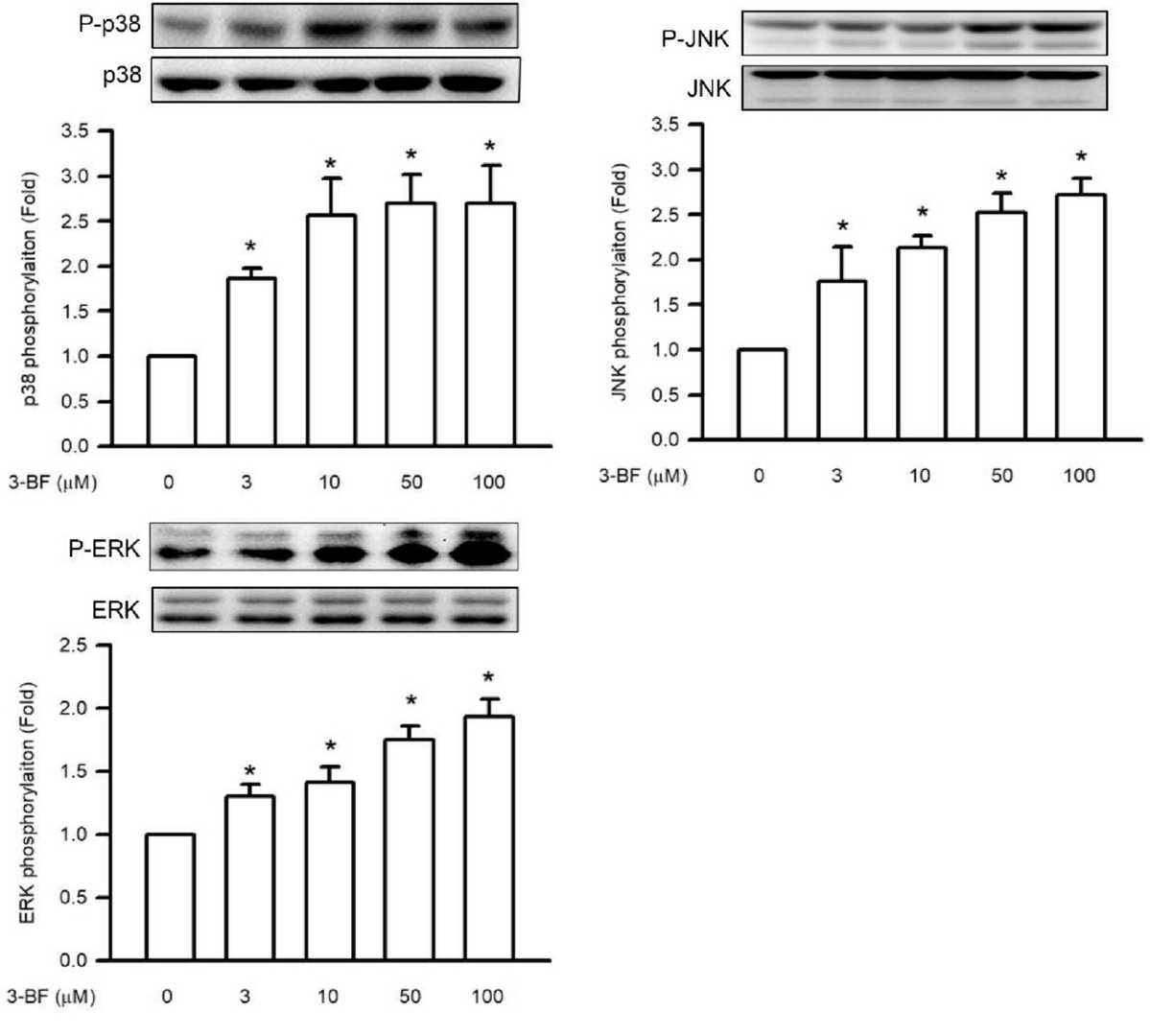 Fig. 1. 3-BrFlu-induced MAPK phosphorylation in SVEC4-10 endothelial cells (Lee C Y, Wu SW, et al., 2024).
Fig. 1. 3-BrFlu-induced MAPK phosphorylation in SVEC4-10 endothelial cells (Lee C Y, Wu SW, et al., 2024).
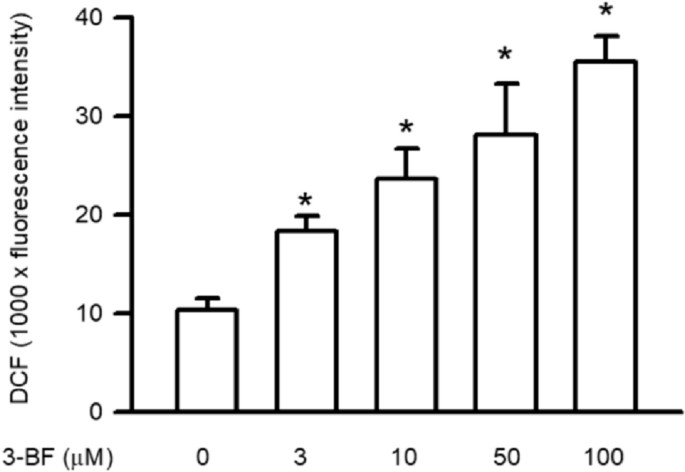 Fig. 2. 3-BrFlu-induced intracellular ROS accumulation in SVEC4-10 endothelial cells (Lee C Y, Wu SW, et al., 2024).
Fig. 2. 3-BrFlu-induced intracellular ROS accumulation in SVEC4-10 endothelial cells (Lee C Y, Wu SW, et al., 2024).
LPA Receptor Expressions and Cell Growth Activity of SVEC4-10 Cells
LPA receptor signaling is crucial in cancer development, influencing cell migration and metastasis within the complex tumor microenvironment, which includes lymphatic endothelial cells. Hypoxia, common in solid tumors, further enhances tumor progression. Yamamoto et al. examined the effects of LPA receptor-mediated signaling on SVEC4-10 lymphatic endothelial cells under hypoxic conditions via in vitro assays. They evaluated changes in cell motility, tube formation, and responses to LPA agonists and antagonists.'
Six G protein-coupled LPA receptors exist, with LPA1, LPA2, and LPA3 belonging to the Edg family, while LPA4 to LPA6 are non-Edg. This study focused on LPA1, LPA2, and LPA3 to explore their roles in regulating hypoxia-induced functions of SVEC4-10 cells. SVEC4-10 cells expressed all LPA receptors except Lpar5 (Fig. 3A). Their growth rate increased under 1% O2 compared to 21% O2 (Fig. 3B), but Lpar1, Lpar2, and Lpar3 expression was lower at 1% O2 (Fig. 3C). LPA did not affect growth under 21% O2 but inhibited it at 1% O2 with 1 μM LPA (Fig. 3D). LPA2 agonist GRI-977143 inhibited growth, while LPA3 agonist (2S)-OMPT enhanced it (Fig. 3E). LPA1 antagonist AM966 increased growth with LPA (1 μM) (Fig. 3F). Thus, LPA1 and LPA2 suppress growth, whereas LPA3 promotes it under hypoxia.
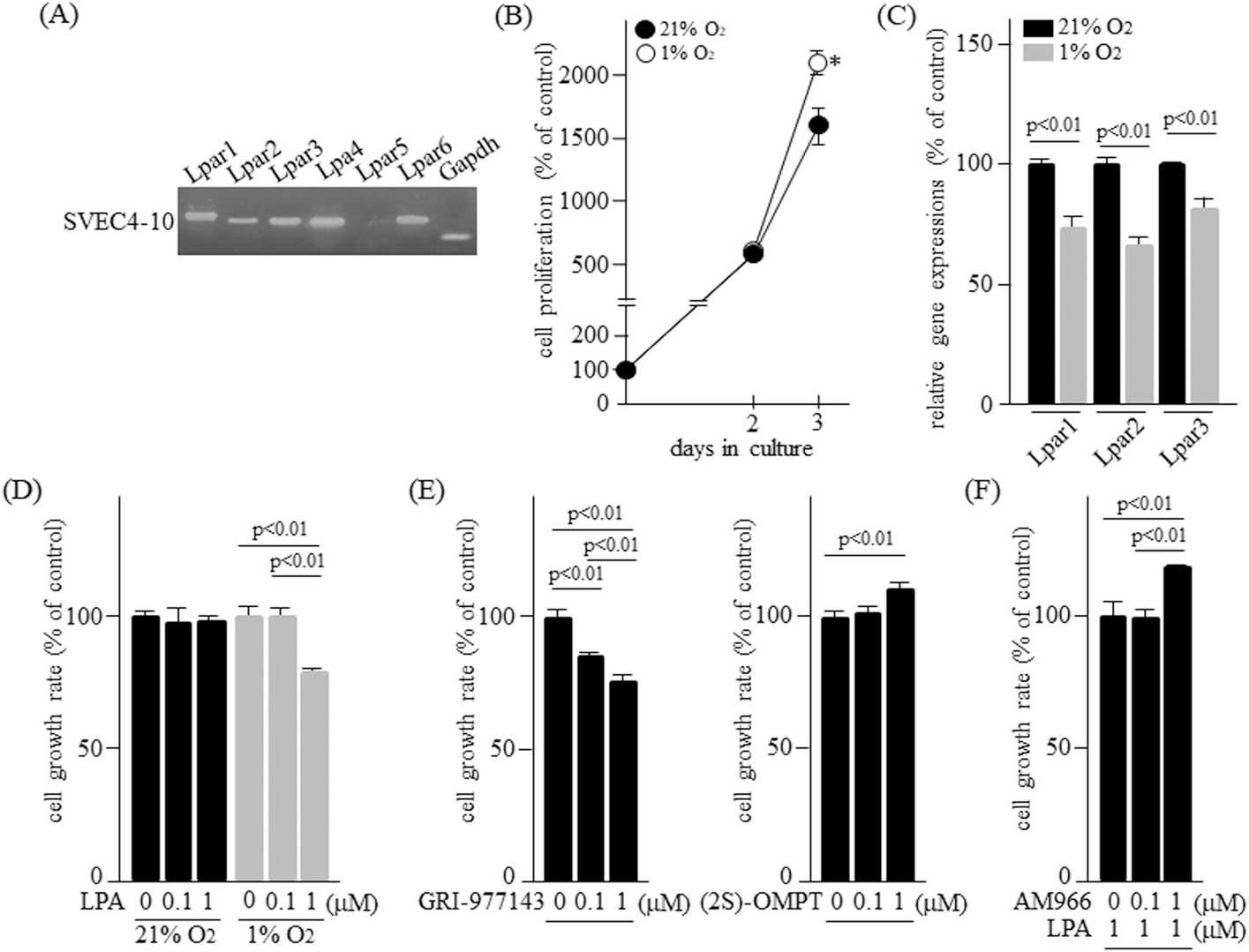 Fig. 3. LPA receptor expressions and cell growth activity of SVEC4-10 cells (Yamamoto M, Takai M, et al., 2024).
Fig. 3. LPA receptor expressions and cell growth activity of SVEC4-10 cells (Yamamoto M, Takai M, et al., 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells