MHEC5-T
Cat.No.: CSC-C2757
Species: Mus musculus (Mouse)
Source: Heart
Morphology: adherent, epithelial-like cells growing as monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cytogenetics: murine flat-moded hexaploid karyotype - 120(96-122) - 2-3r/dmin and 3 centric fusion markers
Viruses: ELISA: reverse transcriptase positive; PCR: SMRV -
MHEC5‑T (Mouse Heart Endothelial cell clone 5) is an immortalized microvascular endothelial cell line isolated from the ventricular wall of the murine heart. Morphologically, MHEC5-T cells have the classic cobblestone appearance and express endothelial cell markers such as CD31 (PECAM-1), VE-cadherin, and von Willebrand factor (vWF). MHEC5-T cells also form capillary-like tubes in angiogenesis assays, and they retain the endothelial functions of nitric oxide production, cytokine responsiveness, and barrier function. For these reasons, MHEC5‑T is a useful immortalized cell line for mechanistic and pharmacological studies.
As MHEC5‑T cells are derived from cardiac endothelium, they are well suited for the study of cardiovascular endothelial physiology, cardiac microvascular remodeling, ischemia-reperfusion injury, and the inflammatory signaling pathways that underlie heart disease. They also serve as a readily available and ethically sourced alternative to primary endothelial cells, which are often limited by donor-to-donor variability, senescence, and isolation challenges. MHEC5-T cells are also frequently used in co-culture systems to study endothelial-cardiomyocyte or endothelial-immune cell interactions, which are important for the investigation of vascular inflammation, leukocyte adhesion, and endothelial permeability.
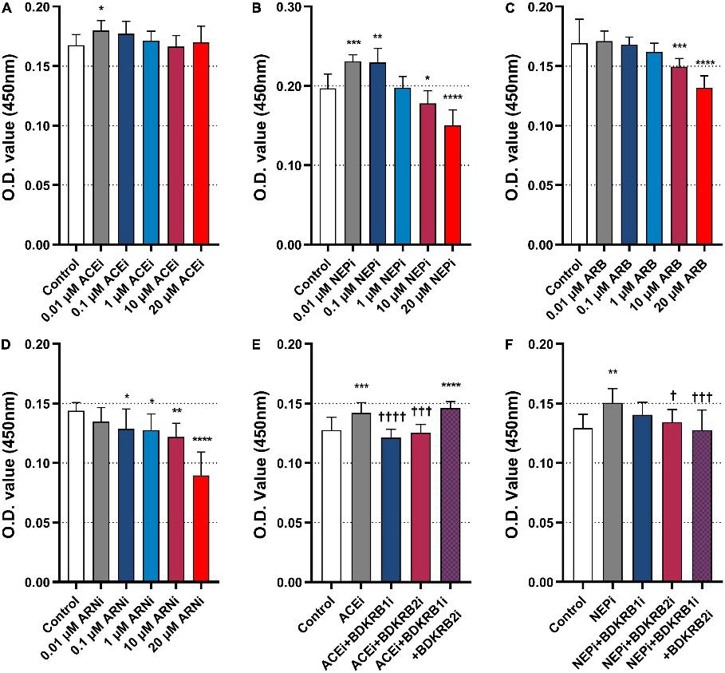
Neprilysin Inhibitors Exert Pro-Arteriogenic Effects Through the Bradykinin Receptor Signaling Pathway
Li's team explored the pleiotropic action of an angiotensin receptor-neprilysin inhibitor (ARNi) in collateral-dependent myocardial perfusion in the rat coronary arteriogenesis model and conducted further analyses to explore the underlying mechanism. In the in vitro study, murine heart endothelial cells (MHEC5-T) were exposed to a neprilysin inhibitor (NEPi) alone or in combination with bradykinin receptor antagonists to determine the effects on cell proliferation.
The results showed that 0.01 μM ACEi significantly increased cell proliferation compared with control (Fig. 1A). In addition, 0.01 μM and 0.1 μM NEPi also significantly increased cell proliferation (Fig. 1B). On the other hand, 10 μM and 20 μM ARB significantly inhibited cell proliferation (Fig. 1C). ARNi (NEPi + ARB) also significantly inhibited cell proliferation in a dose-dependent manner (Fig. 1D). To further examine if the pro-arteriogenic effect of ACEis and ARNis were mediated by the bradykinin signaling pathway, additional tests were performed. ACEi alone promoted cell proliferation, while ACEi combined with BDKRB1i or BDKRB2i inhibited cell proliferation. Nevertheless, ACEi combined with both BDKRB1i and BDKRB2i increased cell proliferation (Fig. 1E). In a similar fashion, NEPi alone promoted cell proliferation, while NEPi combined with BDKRB2i or both BDKRB1i and BDKRB2i inhibited cell proliferation (Fig. 1F).
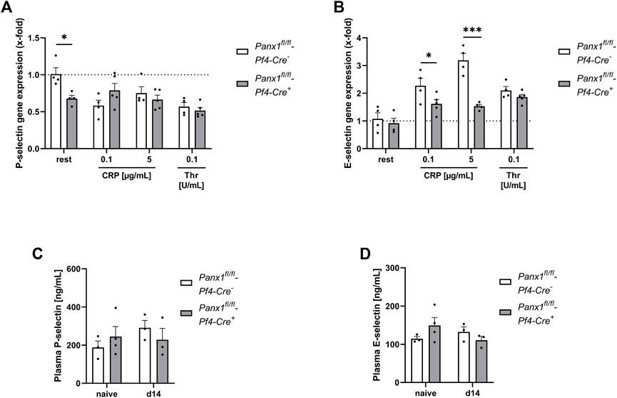
Platelet Panx1 is Important for the Activation of Endothelial Cells
Abdominal aortic aneurysm (AAA) is a common and potentially lethal disease characterized by progressive aortic dilatation and chronic inflammation. Pannexins, particularly Panx1, play a crucial role in inflammation and remodeling. Metz et al. investigated the contribution of platelet Panx1 in AAA progression, given the role of platelets in cardiovascular diseases.
To see if platelet Panx1 affects endothelial cell activation, we tested the activation of MHEC5-T cells (an endothelial cell line) by platelets in vitro. Platelets were activated with CRP or thrombin, and the supernatant was collected. MHEC5-T cells were then incubated with platelet releasates from Panx1-deficient and control mice for 3 hours. They analyzed the gene expression of P-selectin and E-selectin in these cells (Fig. 2A, B). P-selectin expression in MHEC5-T cells changed only with resting, not activated, Panx1-deficient platelets (Fig. 2A). However, E-selectin gene expression was much lower when MHEC5-T cells were incubated with CRP-activated Panx1-deficient platelet supernatant compared to controls (Fig. 2B). This suggests that platelet Panx1 is important for endothelial cell activation, as shown by the expression of adhesion molecules like P- and E-selectin (Fig. 2A, B). They also checked plasma levels of soluble P-selectin and E-selectin in PPE mice using platelet-specific Panx1-deficient mice and controls. No differences were found 14 days after PPE infusion in mouse aorta between groups (Fig. 2C, D).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells