MC3T3-E1
Cat.No.: CSC-C3037
Species: Mus musculus (Mouse)
Source: Bone
Morphology: Fibroblast-like
Culture Properties: adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
MC3T3-E1 is a mouse osteoblastic cell line that is fibroblast-like preosteoblasts, which can be induced to terminally differentiate into mature osteoblasts in vitro. This cell line is used as an in vitro model for osteoblast differentiation and bone formation, and is commonly used in experiments studying extracellular matrix (ECM) signaling. MC3T3-E1 cells can express osteoblast markers alkaline phosphatase (ALP), osteocalcin (OCN), and collagen, and can deposit mineralized extracellular matrix upon induction with ascorbic acid and inorganic phosphate. Certain subclones of the MC3T3-E1 cell line (including Subclone 4 and 14) have a high osteogenic potential. The MC3T3-E1 cell line has proven to be useful for studies of bone-related disease such as osteoporosis, bone tumors and bone regeneration, and it is also commonly used in 3D cell culture.
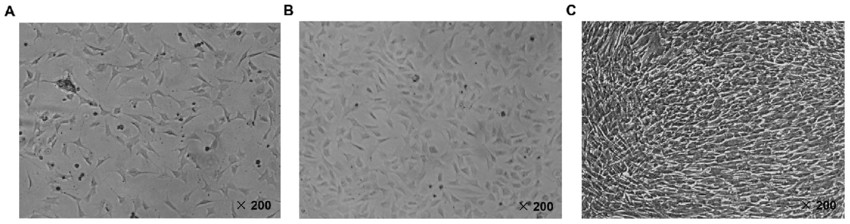 Fig. 1. Identification of MC3T3-E1 mouse osteoblast cell line. The MC3T3-E1 cells were observed using an inverted fluorescence microscope at x200 magnification following culture for (A) 24 h, (B) 48 h and (C) 72 h, respectively (Zhu Z G, Xie Q P, et al., 2018).
Fig. 1. Identification of MC3T3-E1 mouse osteoblast cell line. The MC3T3-E1 cells were observed using an inverted fluorescence microscope at x200 magnification following culture for (A) 24 h, (B) 48 h and (C) 72 h, respectively (Zhu Z G, Xie Q P, et al., 2018).
MC3T3-E1 Cell Death Associated with the Accumulation of TAGE
Diabetes is a known risk factor for osteoporotic fractures, and it has been suggested that the hyperglycemia-induced formation of advanced glycation end-products (AGEs) plays a causative role. The AGEs, which are cytotoxic, can also inhibit osteoblast function. Sakasai-Sakai et al. investigated the cytotoxic effect of intracellular glyceraldehyde (GA) and GA-derived toxic AGEs (TAGE) in the MC3T3-E1 osteoblast cell line, and the mechanisms by which GA and TAGE affect osteoblasts.
They first tested the effect of GA on mouse osteoblastic MC3T3-E1 cells using a cell viability assay, as osteoblast death is a key cause of osteoporosis. Cell viability decreased in a dose-dependent manner with increasing GA concentration from 1 mM GA (Fig. 1A). The GA reacts with proteins and forms TAGE that can damage cell functions. Next, they checked the accumulation of TAGE in GA-treated MC3T3-E1 cells by slot blot analysis with an anti-TAGE antibody. TAGE level significantly increased with 2 mM GA (Fig. 1B). Western blot analysis revealed that 2 mM GA increased several TAGE-modified protein bands (Fig. 1C). They used AG, an inhibitor of AGE formation that can react with aldehydes. AG pretreatment decreased GA-induced TAGE accumulation (Fig. 1B) and reduced TAGE band intensities (Fig. 1C). In addition, AG pretreatment inhibited GA-induced cell death (Fig. 1D), which suggests that TAGE accumulation is correlated with MC3T3-E1 cell death.
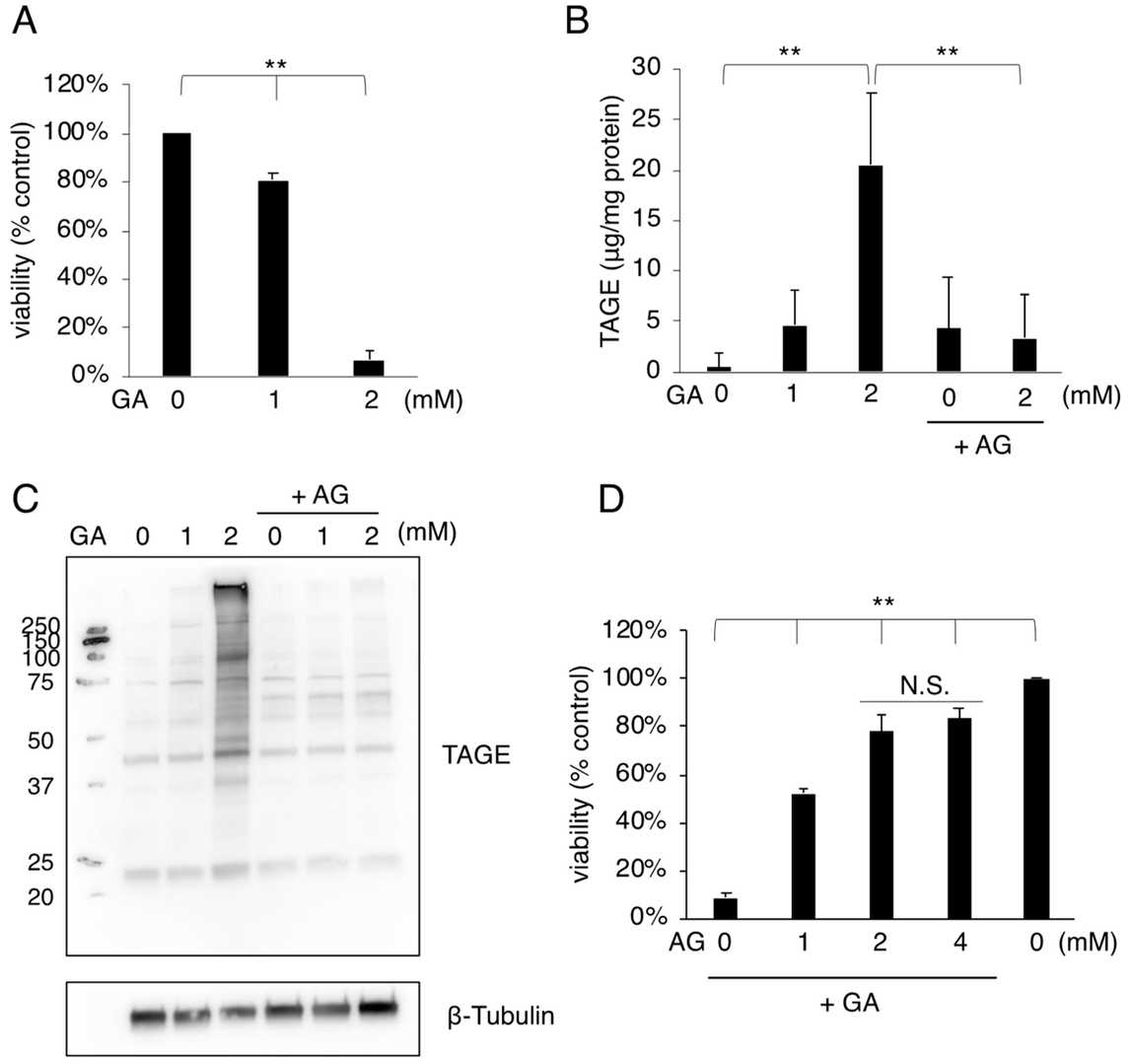 Fig. 1. Cell death and TAGE accumulation were induced in MC3T3-E1 cells treated with GA (Sakasai-Sakai A, Takata T, et al., 2022).
Fig. 1. Cell death and TAGE accumulation were induced in MC3T3-E1 cells treated with GA (Sakasai-Sakai A, Takata T, et al., 2022).
Apoptosis-Promoting Properties of miR-3074-5p in MC3T3-E1 Cells under Iron Overload Conditions
Iron overload contributes to osteoporosis by triggering apoptosis in osteoblasts. However, the regulatory mechanism of miRNA on osteoblast apoptosis under iron overload is unclear. Using next-generation sequencing analysis to examine the miRNA expression profiles of iron-overloaded MC3T3-E1 cells, Feng's team investigated the mechanism by which miR-3074-5p regulates apoptosis in MC3T3-E1 cells under iron overload.
Differentially expressed miRNAs in MC3T3-E1 cells treated with 1.8 mM FAC for 24 h were identified by miRNA next-generation sequencing (Fig. 2a). The expression of miR-3074-5p was significantly increased in iron-overloaded MC3T3-E1 cells. qRT-PCR results showed that the expression of miR-3074-5p was significantly higher in FAC-treated cells (Fig. 2b). Transfection of a miR-3074-5p inhibitor rescued FAC-induced upregulation (Fig. 2c). These results indicated the potential effect of miR-3074-5p under iron overload. MC3T3-E1 cells were transfected with a miR-3074-5p mimic or inhibitor to perform gain- and loss-of-function experiments. CCK-8 assays and Annexin V-FITC/PI staining confirmed that the miR-3074-5p mimic significantly increased the cell inhibition and apoptosis rates of MC3T3-E1 cells under iron overload, while the inhibitor significantly decreased the cell inhibition and apoptosis rates (Fig. 3a, 3b). Thus, miR-3074-5p promotes the apoptosis of iron-overloaded MC3T3-E1 cells.
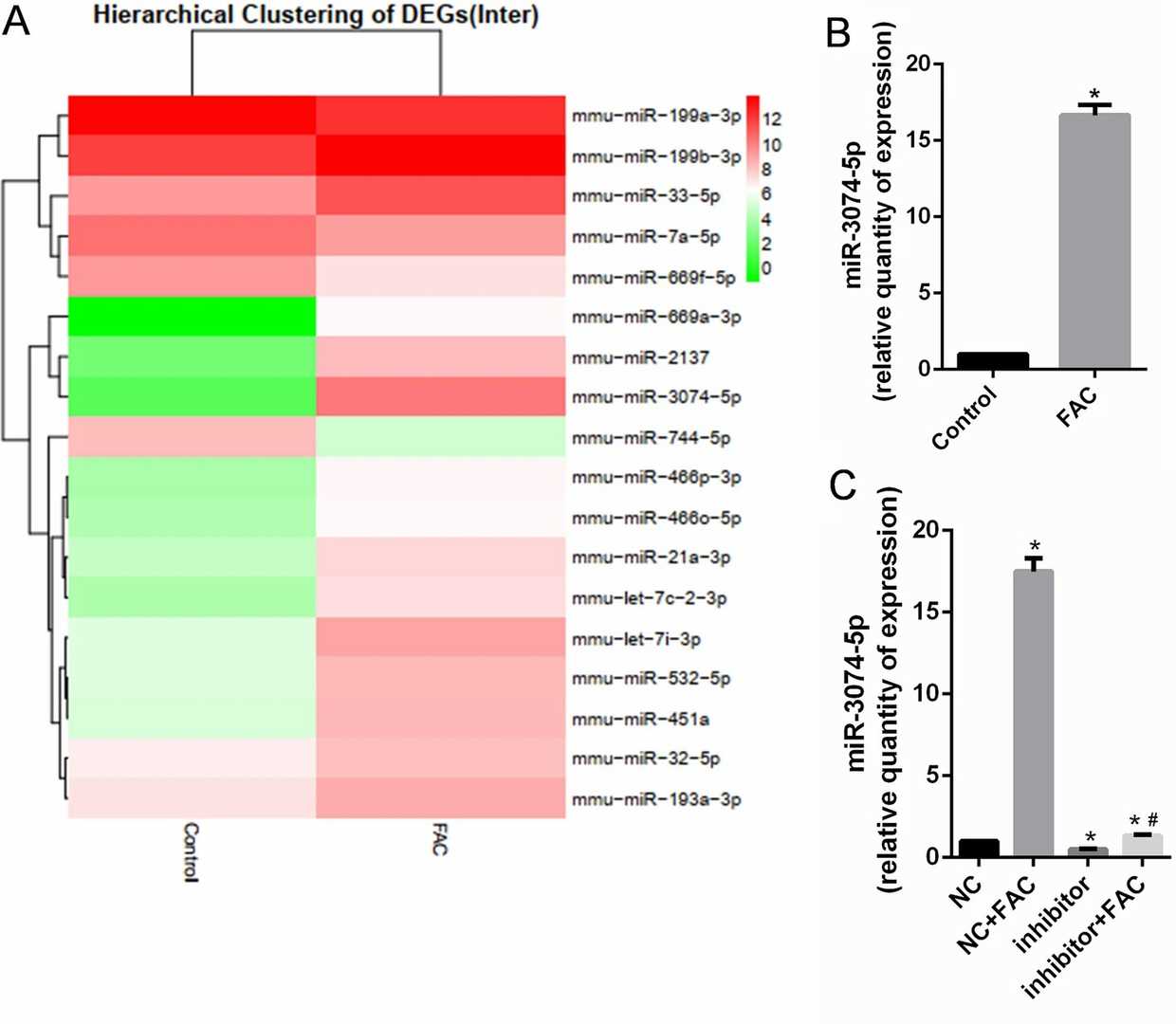 Fig. 2. miR-3074-5p expression was increased in MC3T3-E1 cells under iron overload conditions (Feng Y, He P Y, et al., 2021).
Fig. 2. miR-3074-5p expression was increased in MC3T3-E1 cells under iron overload conditions (Feng Y, He P Y, et al., 2021).
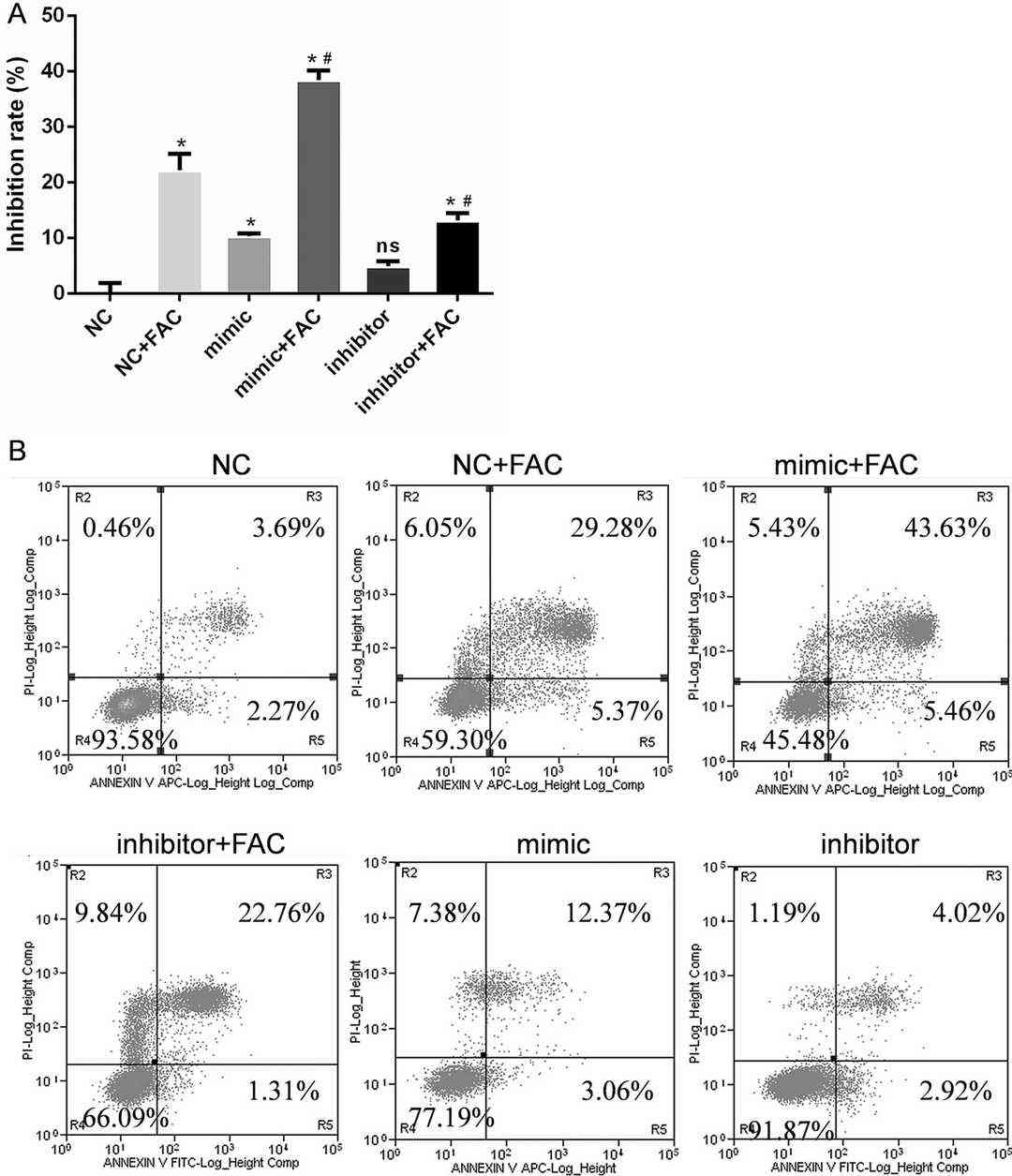 Fig. 3. miR-3074-5p mediated the apoptosis of MC3T3-E1 cells under iron overload conditions (Feng Y, He P Y, et al., 2021).
Fig. 3. miR-3074-5p mediated the apoptosis of MC3T3-E1 cells under iron overload conditions (Feng Y, He P Y, et al., 2021).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells