LS180
Cat.No.: CSC-C9208W
Species: Homo sapiens (Human)
Source: Intestine; Colon
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The LS180 cell line was originally established from colorectal tissue of a 58-year-old Caucasian female patient with Dukes B stage colon adenocarcinoma. LS180 cells have an epithelial-like morphology with vacuolar structures. They are tumorigenic and can form tumors in nude mice. The modal chromosome number of LS180 cells is 45 and their chromosome number varies between 42 and 47 chromosomes.
LS180 cells have been used in numerous studies on colorectal cancer including studying the roles of the m6A methyltransferase KIAA1429, RASSF10 and ABHD5 in this type of cancer. The cell line has also been used for screening the antitumor activities of new types of anticancer drugs including derivatives of sophoridine and honokiol. Additionally, the LS180 cell line has facilitated investigations into the regulation of signaling pathways in colorectal cancer, such as the P53 and S100P pathways.
Gene and Protein Expression Analysis of PD-L1 and the EMR Proteins in LS180 Cells
Colorectal cancer (CRC) is the third most prevalent and the second most fatal cancer in the world. The currently available treatment options of surgery, chemotherapy, and radiotherapy have poor clinical benefits, thus necessitating the development of new therapeutic approaches. Programmed cell death protein ligand-1 (PD-L1), an immune checkpoint protein that is upregulated in several cancer cells, plays an important role in immune evasion by cancer cells.
Kobori et al. investigated whether ezrin, radixin, and moesin (ERM) proteins, which act as scaffolds linking plasma membrane proteins to the actin cytoskeleton, influence PD-L1 expression on the cell surface of human colorectal adenocarcinoma cells (LS180). They initially examined the gene expression levels of PD-L1, ezrin, radixin, and moesin in LS180 and Caco-2 cells. RT-qPCR analysis revealed higher PD-L1 gene expression in LS180 cells compared to that in Caco-2 cells (Fig. 1a). Ezrin gene expression was comparable between the two cell lines (Fig. 1b). Radixin gene expression was lower in LS180 cells compared to that in Caco-2 cells (Fig. 1c). Moesin gene expression was only detected in LS180 cells but not in Caco-2 cells (Fig. 1d), which was consistent with the previous report that Caco-2 cells do not express moesin. Therefore, the authors used LS180 cells for the subsequent experiments to examine the role of ERM proteins in PD-L1 expression. Western blot analysis also showed detectable protein expression levels of PD-L1 in addition to ezrin, radixin, and moesin in the whole-cell lysates of LS180 cells (Fig. 1e).
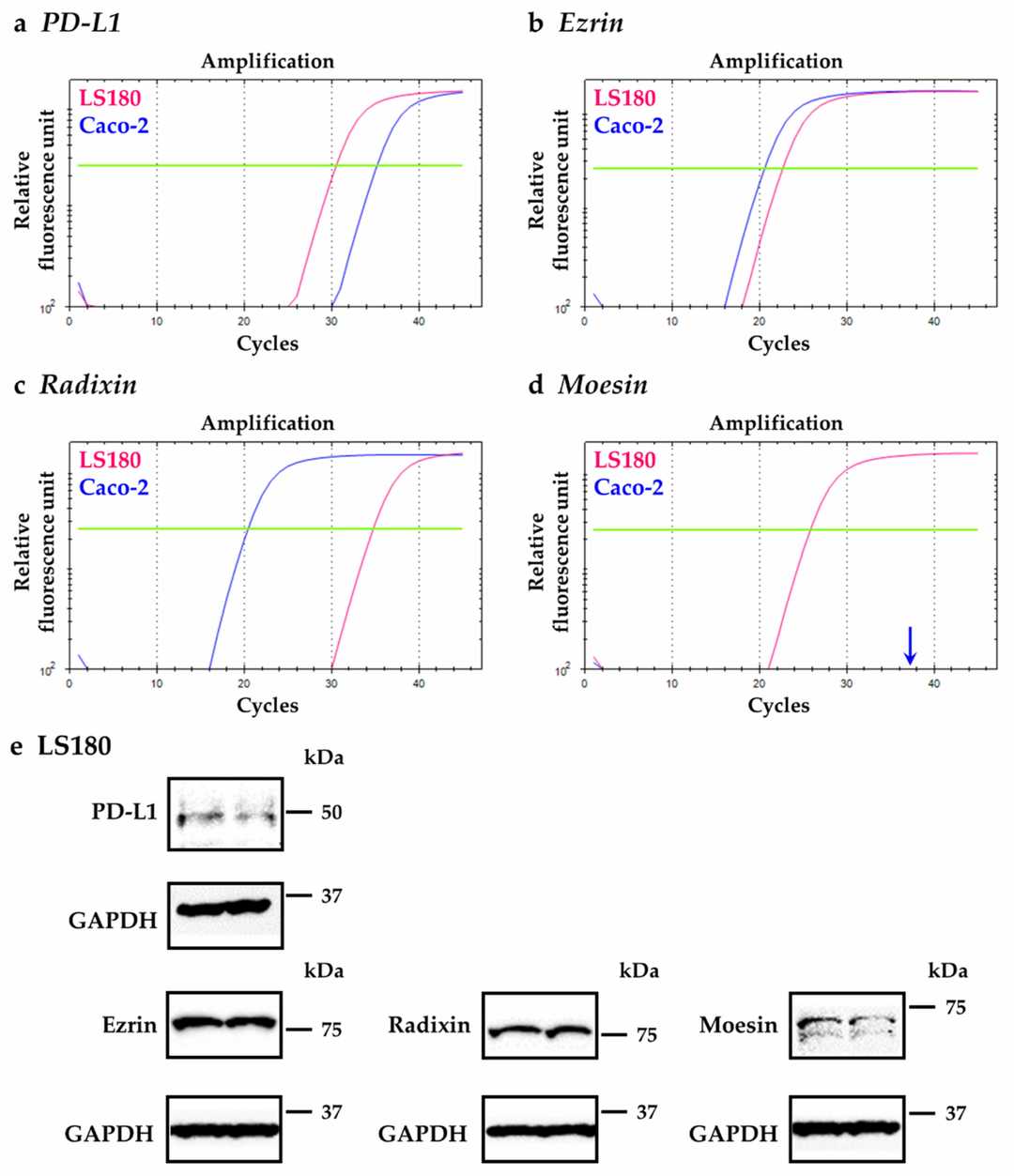 Fig. 1. Gene and protein expression profiles of programmed cell death ligand-1 (PD-L1), ezrin, radixin, and moesin (ERM) in LS180 cells and Caco-2 cells (Kobori T, Tanaka C, et al., 2021).
Fig. 1. Gene and protein expression profiles of programmed cell death ligand-1 (PD-L1), ezrin, radixin, and moesin (ERM) in LS180 cells and Caco-2 cells (Kobori T, Tanaka C, et al., 2021).
Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells
Colorectal cancer is one of the most frequent tumors in developed countries. Hydroxytyrosol, a powerful antioxidant, is able to exert control over oxidative stress, proliferation inhibition, and apoptosis induction. In the present work, the modulation induced by hydroxytyrosol on apoptotic gene expression (BAX, BCL2, CASP3, P53, PPARG, and NFE2L2) and antioxidant enzyme activity in human colorectal LS180 cancer cells was studied. Methods include treating LS180 cells with hydroxytyrosol, analyzing gene expression via real-time PCR, and measuring enzyme activity calorimetrically. The study found that Hydroxytyrosol causes a significant up-regulation of BAX expression at all the tested concentrations (Fig. 2A). BCL2 expression significantly increased (Fig. 2B). BAX:BCL2 ratio (Fig. 2C) and CASP3 expression (Fig. 2D) are significantly higher in all treated groups. P53 expression is not significantly altered after hydroxytyrosol treatment (Fig. 3A), while NFE2L2 expression is significantly down-regulated at 50 μM (Fig. 3B). Hydroxytyrosol treatment results in a significant down-regulation of PPARG expression at all the concentrations used (Fig. 3C).
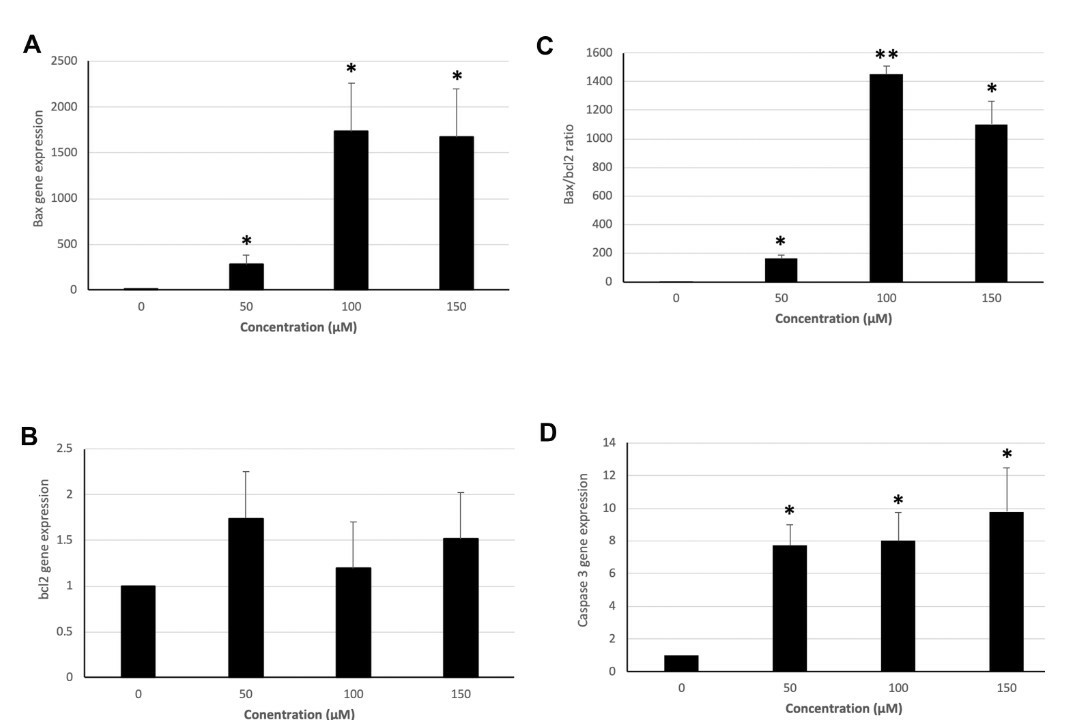 Fig. 2. Expression of BAX, BCL2, and CASP3 and BAX:BCL2 ratio in groups treated with 50, 100, and 150 μM hydroxytyrosol and controls in the LS180 cell line (Hormozi M, Marzijerani A S, et al., 2020).
Fig. 2. Expression of BAX, BCL2, and CASP3 and BAX:BCL2 ratio in groups treated with 50, 100, and 150 μM hydroxytyrosol and controls in the LS180 cell line (Hormozi M, Marzijerani A S, et al., 2020).
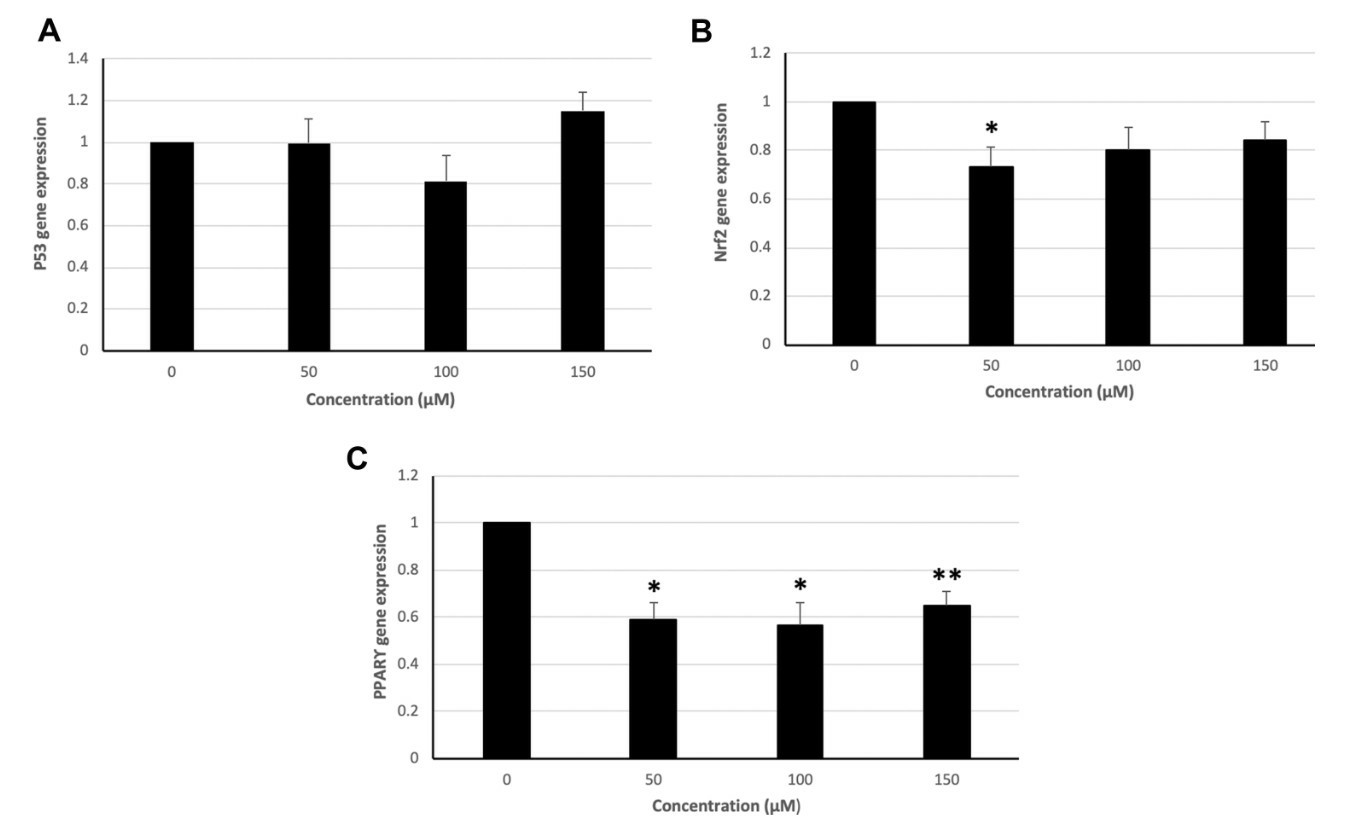 Fig. 3. Expression of P53, NFE2L2, and PPARG genes in groups treated with 50, 100, and 150 μM hydroxytyrosol and controls in the LS180 cell line (Hormozi M, Marzijerani A S, et al., 2020).
Fig. 3. Expression of P53, NFE2L2, and PPARG genes in groups treated with 50, 100, and 150 μM hydroxytyrosol and controls in the LS180 cell line (Hormozi M, Marzijerani A S, et al., 2020).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells