GPE 86
Cat.No.: CSC-6256W
Species: Mus musculus (Mouse)
Source: Embryo
Morphology: continuous culture, grown as monolayer, morphology fibroblast
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Tissue: kidney, embryonic
GPE‑86 (sometimes written as GP+E‑86) is a retroviral packaging cell line originally constructed from NIH‑3T3 mouse embryonic fibroblasts stably transfected with Moloney murine leukemia virus (M‑MLV) gag, pol and ecotropic env. The ecotropic env is constitutively expressed, thereby restricting virus from GPE-86 cells to mouse and rat cell lines and primary cells expressing the mCAT1 receptor.
The predominant use of GPE‑86 is to generate high‑titer ecotropic retrovirus to transduce murine target cells. The line is routinely transfected with a retroviral vector (MSCV‑based GFP or puromycin‑resistance constructs, for example) and supernatants containing infectious particles are harvested for the purpose of integrating the transgene into murine cell lines, primary hematopoietic progenitors, or ES cells. GPE-86's ecotropic specificity is also exploited in T‑cell receptor (TCR) and chimeric antigen receptor (CAR) studies in which GPE‑86 produced virus is used to create murine TCR-retrogenic mice or CAR-T cells for immunology studies. GPE-86 is a common positive control for packaging‑efficiency assays, envelope‑pseudotyping studies, and for safety screening of RCR formation.
GPE‑86 has also been put to other uses including modified for gene delivery in a non-traditional manner by RNAi‑mediated silencing of its env component, allowing the generation of pantropic retroviruses to transduce human CD34⁺ progenitors. GPE-86's stable nature, ease of culture, and well-studied safety profile make it a foundational tool for the production of murine retroviral vectors and functional genomics.
Influence of ENI Platform'S Applied Voltages on Cell Viability
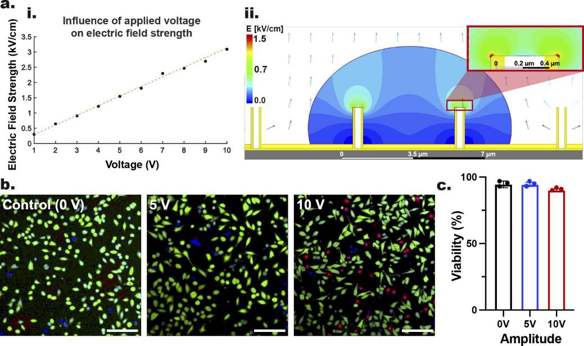
Nanoinjection efficiently delivers intracellular cargo with minimal cell perturbation, overcoming issues like safety, genotoxicity, and poor versatility in conventional methods. Shokouhi et al. proposed a non-viral, low-voltage, reusable electroactive nanoinjection (ENI) platform using conductive nanotubes to achieve precise biomolecular delivery and gene silencing.
To study the impact of nanoscale EP on cell viability via the ENI platform, they applied 5 V and 10 V monophasic square-wave pulses with a low-voltage generator. The electrical parameters were: 20 Hz frequency, 400 µs pulse-width, 600 cycles, and 5 ns rise/fall time. Post-nanoscale-EP, GPE-86 cells were stained with Hoechst 33,342, PI, and FDA to indicate nuclei, dead, and live cells, respectively. Confocal microscopy images and quantification (Fig. 1b, c) showed 94.5±1.5% viability at 5 V, similar to non-electroporated cells (94.5±2.5%), and slightly lower at 10 V (90.1±0.9%). This indicates minimal cell damage during nanoscale-EP, with most cells recovering. To avoid further viability reduction, they set 10 V, 400 µs, 20 Hz, 600 cycles as the threshold parameters for our ENI platform.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells