GC-1 spg
Cat.No.: CSC-C9183W
Species: Mus musculus (Mouse)
Source: Testis
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The GC-1 spg cell line originates from the mouse testis and represents Type B spermatogonia which remains undifferentiated during spermatogenesis. GC-1 spg cells function primarily to drive spermatogenesis by supporting both spermatogonia proliferation and differentiation and the development of primary spermatocytes. The culture medium's nutrients and growth factors like testosterone and melatonin control GC-1 spg cell proliferation and differentiation in vitro. GC-1 spg cells exhibit two testis-specific isoforms of cytochrome c and lactate dehydrogenase C4 which demonstrate their similarity to spermatocytes as they exist in the body. GC-1 spg cells express the somatic cell marker WT1 but they do not show expression of the germ cell marker.
These cells serve as crucial models for researching male reproductive health alongside the regulatory processes of spermatogenesis and the development of associated disorders including infertility. For example, scientists analyze this cell line using transcriptomics, proteomics, and epigenetics to explore how essential genes and signaling pathways function during spermatogenesis. Researchers can investigate the role of genes in spermatogenesis through either transfection of specific genes or application of RNA interference techniques.
DEP Induces Oxidative Stress in GC-1 spg Cells
DEP particles trigger oxidative stress and inflammation which negatively affects male reproductive health by disrupting spermatogenesis. Shi's team investigates how DEP triggers oxidative stress in GC-1 spg cells by analyzing the role of reactive oxygen species (ROS) buildup and the Keap1-Nrf2 signaling pathway.
They investigated oxidative stress contributions to DEP-induced cytotoxic effects on GC-1 spg cells by measuring cellular oxidative damage through biochemical indices. After DEP exposure, DCFH-DA fluorescence intensity increased significantly in a dose-dependent manner, showing that DEP increased ROS formation in GC-1 spg cells (Fig. 1a and b). Excess ROS can damage cell membranes and cause mitochondrial injury. We assessed mitochondrial membrane potential (MMP) using JC-1 dye. As DEP concentration increased, J-monomer fluorescence increased, while J-aggregate fluorescence decreased. Quantitative analysis showed a significant decrease in the J-aggregate/J-monomer ratio in DEP-treated cells, indicating mitochondrial depolarization (Fig. 1c and d). MDA is a marker for lipid peroxidation. In GC-1 spg cells treated with DEP, MDA concentration increased with DEP concentration (Fig. 1e). They also measured GSH levels, which decreased with DEP treatment (Fig. 1f). SOD activity was also reduced in DEP-exposed cells. The results show that DEP treatment caused oxidative damage and stimulated antioxidant defenses.
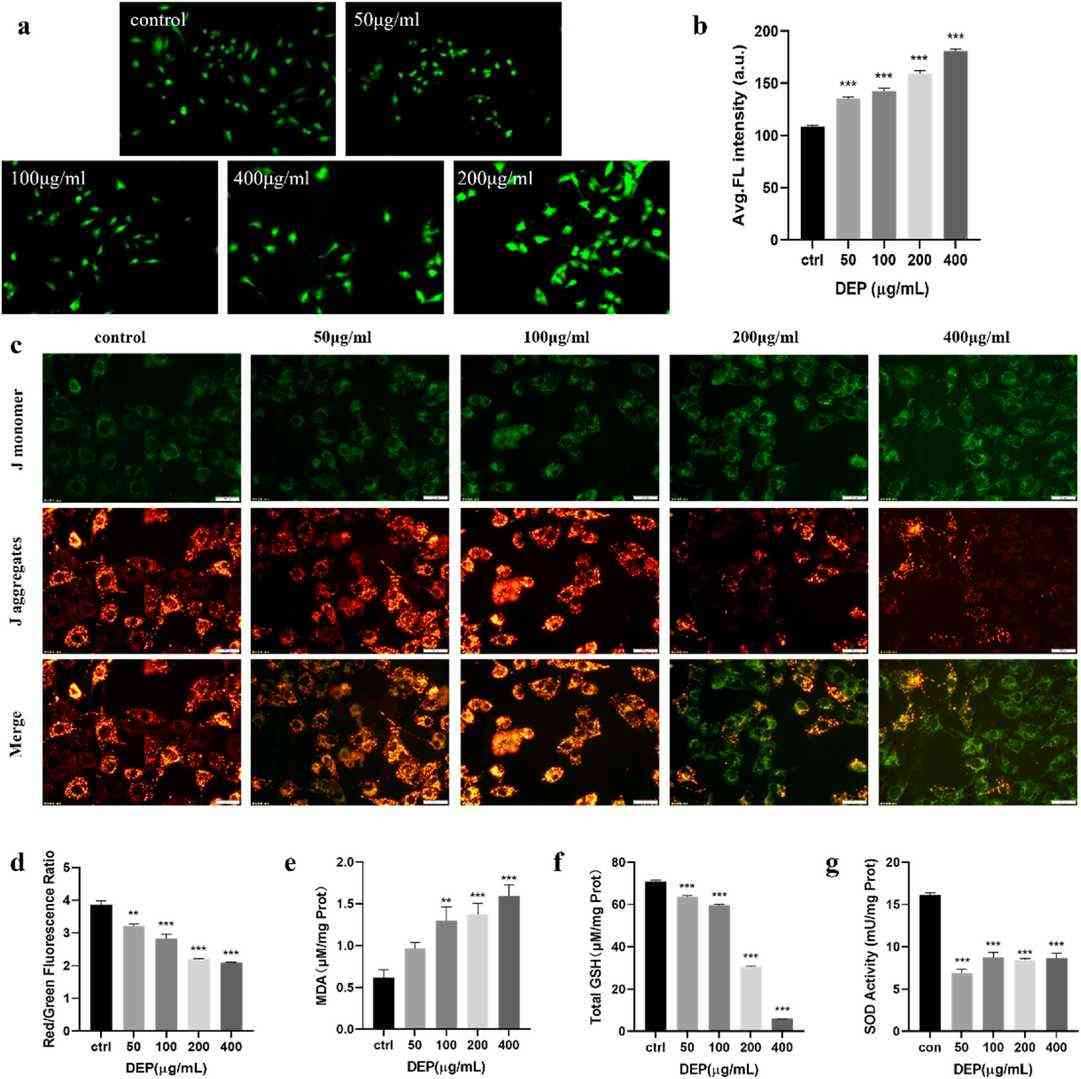 Fig. 1. DEP induced oxidative stress and mitochondrial damage in GC-1spg cell (Shi J, Tian F, et al., 2024).
Fig. 1. DEP induced oxidative stress and mitochondrial damage in GC-1spg cell (Shi J, Tian F, et al., 2024).
ZnO NPsInduces Apoptosis and Autophagy of Mouse GC-1 spg Cells
Zinc oxide nanoparticles (ZnO NPs), widely used for their unique properties, have raised health concerns due to their potential to induce reproductive toxicity, particularly affecting spermatogenesis. Yang's team investigated how oxidative stress contributes to ZnO NPs-induced apoptosis and autophagy in mouse GC-1 spg cells, shedding light on mechanisms underlying reproductive toxicity and potential spermatogenesis failure.
ZnO NPs inhibited GC-1 spg cell viability in a dose-dependent manner (Fig. 2A). They increased protein levels of cleaved Caspase-8, Caspase-3, and Bax, while reducing Bcl-2 levels (Fig. 2B and C). ZnO NPs also raised early (AnnexinV+/PI?) and late apoptosis (AnnexinV+/PI+) cell counts (Fig. 2D and E), indicating apoptosis induction. ZnO NPs significantly raised LC3 content, LC3-II/LC3-I ratio, and ATG 5, Beclin 1 protein levels (Fig. 3A and B). TEM revealed few autophagosomes in control cells but many in ZnO NPs- and starvation-treated cells, which showed organelle degradation like mitochondria and endoplasmic reticulum (Fig. 3C), indicating autophagy induction.
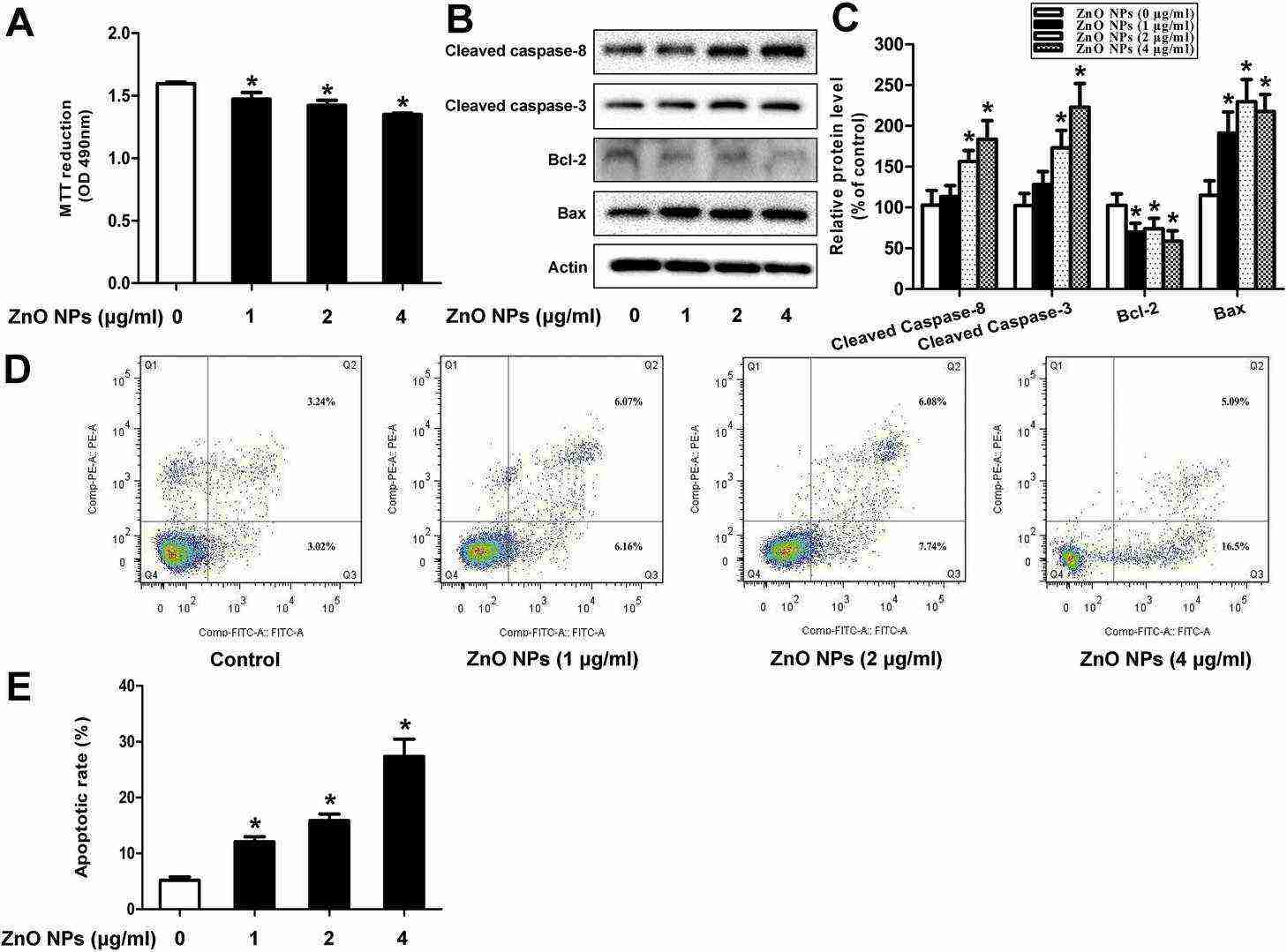 Fig. 2. ZnO NPs induces apoptosis of mouse GC-1 spg cells (Yang D, Zhang M, et al., 2020).
Fig. 2. ZnO NPs induces apoptosis of mouse GC-1 spg cells (Yang D, Zhang M, et al., 2020).
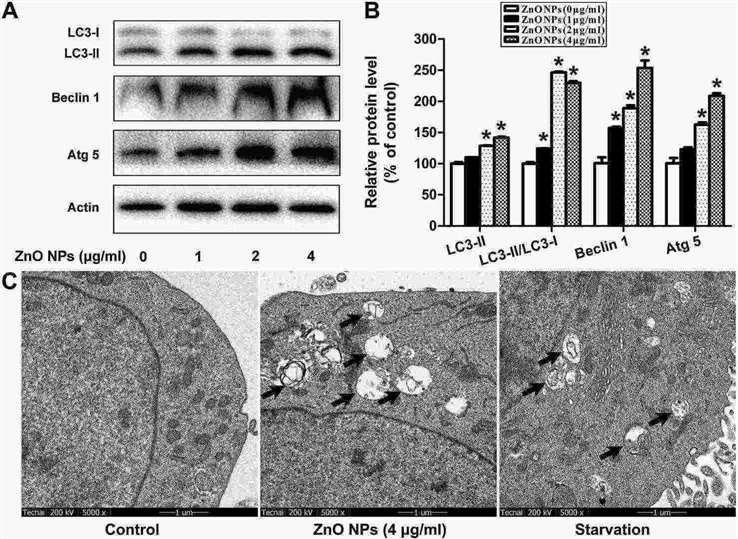 Fig. 3. ZnO NPs induces autophagy of mouse GC-1 spg cells (Yang D, Zhang M, et al., 2020).
Fig. 3. ZnO NPs induces autophagy of mouse GC-1 spg cells (Yang D, Zhang M, et al., 2020).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells