Clone M-3; Cloudman S91 melanoma
Cat.No.: CSC-C9359L
Species: Mus musculus (Mouse)
Source: Skin
Morphology: epithelial
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Strain: (C X DBA)F1
Tumorigenecity: yes, in C57BL mice
Production: melanin
Histopathology: melanoma; melanotic
Note: tested and found negative for ectromelia virus (mousepox)
Clone M-3 (Cloudman S91 melanoma) is an adherent transplantable murine melanoma cell line which was isolated from a spontaneous eye orbit melanoma in DBA/2 mouse in the 1930's and cloned in the 1950's. It is one of the oldest melanoma cell lines that have been established and is extensively characterized. Clone M-3 cells are adherent in culture and have a fibroblast-like morphology, which is typical of melanoma cells. They are also positive for melanin granules. Cells are highly sensitive to α-melanocyte-stimulating hormone (α-MSH), which strongly upregulates melanogenesis through the cAMP signaling pathway, which has made them one of the most popular models for pigment biology. Its strong tumorigenicity in immunocompetent DBA/2 mice is useful in cancer biology, immunology and therapy. Clone M-3 is commonly used in research on melanin synthesis, preclinical drug testing, and tumor-host interactions.
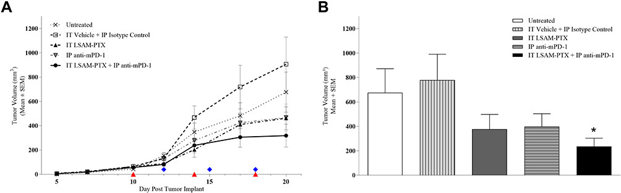
Intratumoral Treatment of Melanoma Tumors with Large Surface Area Microparticle Paclitaxel and Synergy with Immune Checkpoint Inhibition
Melanoma treatment often requires a combination of therapies. Intratumoral drug delivery systems offer a way to directly target tumors. Maulhardt et al. evaluated the effects of intratumoral large surface area microparticle paclitaxel (LSAM-PTX) alone and in combination with systemic anti-PD-1 therapy in a murine melanoma model.
The original melanocyte cell line Clone M-3 [Cloudman S91 melanoma] was passaged once in vivo, resulting in clone M3(Z1) and implanted into the left mammary fat pad. Ten days after tumor implant, animals were block-randomized into five groups (n=8/group) with group mean TV = 56.8 mm3 (range 42.8–66.3 mm3) and treatments were initiated. On Day 20, the IT LSAM-PTX-treated and IP anti-mPD-1-treated groups showed noticeable nonsignificant inhibition of TV compared to the vehicle-isotype control group and TV was further reduced in the group treated with the combination of IT LSAM-PTX + IP anti-mPD-1 (Fig. 1a). When TV and tumor weight was measured following necropsy on Day 20, the combined treatment of IT LSAM-PTX and IP anti-mPD-1 resulted in significant (p < 0.05) reduction in both TV (Fig. 1b) and tumor weight compared to control-treated animals.
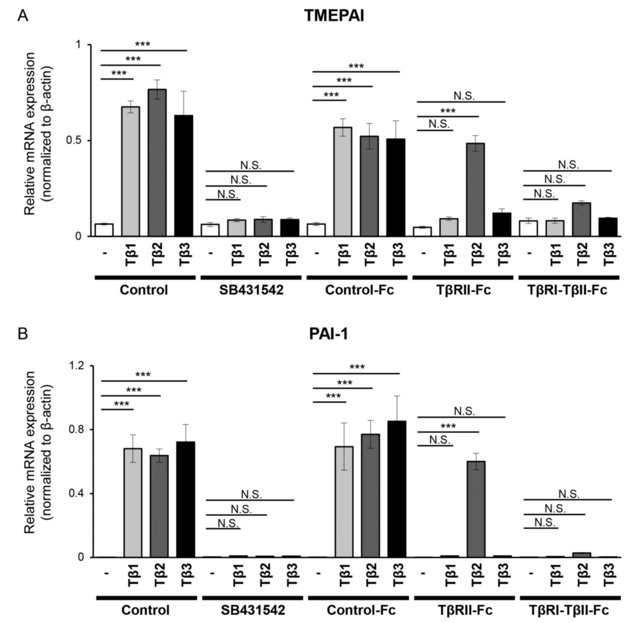
TβRI-TβRII-Fc Chimeric Receptor Efficiently Suppresses TGF-β Signals in Melanoma Cells
Melanoma is an aggressive cancer originating from melanocytes. Transforming growth factor‑β (TGF‑β) promotes melanoma progression by inducing the epithelial‑mesenchymal transition (EMT) and creating a tumor-favorable environment. Three TGF‑β isoforms signal through SMAD pathways, therefore, all TGF-β isoforms should be targeted. Kodama et al. validated the activity of Fc chimeric receptors for TGF-β isoforms in melanoma.
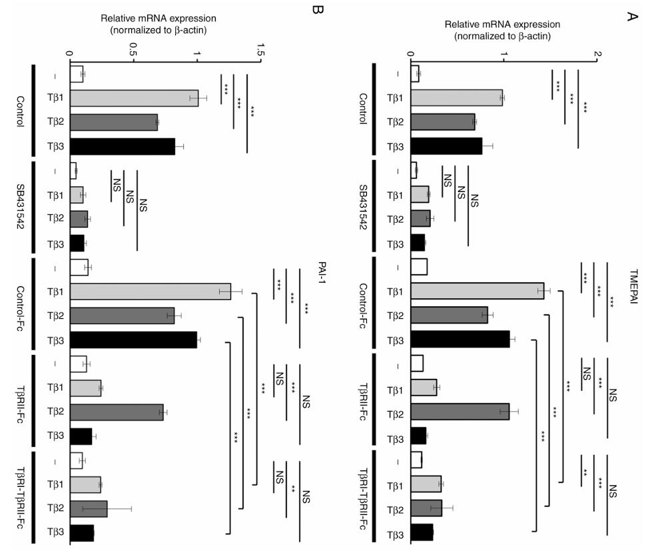
In this study, B16 melanoma cells were used. 293T cells were transfected with vectors expressing Control-Fc, TβRII-Fc, or TβRI-TβRII-Fc chimeric receptors and the accumulation of these chimeric proteins in the conditioned media was validated by immunoblotting. B16 cells were incubated with the conditioned media of 293T cells expressing Control-Fc, TβRII-Fc, or TβRI-TβRII-Fc chimeric receptors in the presence or absence of TGF-β1, -β2, or -β3. Expression of the TGF-β-responsive genes TMEPAI and PAI-1 were evaluated by RT-qPCR. TGF-β isoforms upregulated TMEPAI (Fig. 2A) and PAI-1 (Fig. 2B) expression. Treatment of cells with TβRI kinase inhibitor SB431542 decreased the expression of these genes to the background level (Fig. 2). As expected, Control-Fc did not abrogate TGF-β-induced TMEPAI and PAI-1 expression. TβRII-Fc blocked TGF-β1 and TGF-β3 but not TGF-β2-induced expression of both genes (Fig. 2). In contrast, TβRI-TβRII-Fc significantly inhibited expression of both TMEPAI and PAI-1 genes induced by all three TGF-β isoforms, demonstrating its capacity to block TGF-β signals in melanoma model. To confirm these results, the same experiments were repeated in another melanoma cell line Clone M3. Clone M3 cells were also responsive to TGF-β isoforms, and TGF-β treatment increased expression of TMEPAI (Fig. 3A) and PAI-1 (Fig. 3B). Incubation with TβRI-TβRII-Fc decreased TMEPAI and PAI-1 expression induced by all three TGF-βs in comparison with Control-Fc, demonstrating that TβRI-TβRII-Fc abrogated TGF-β signals in two melanoma cell types.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells