BALB/3T12-3
Cat.No.: CSC-C9338L
Species: Mus musculus (Mouse)
Source: Embryo
Morphology: fibroblast
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Strain: BALB/c
Virus Susceptibility: herpes simples; vesicular stomatitis(Indiana); SV40
Tumorigenecity: yes
Histocompatibility: H-2d
Shipping Condition: Room Temperature
The BALB/3T12-3 cell line originates from mouse embryonic fibroblasts which were extracted from BALB/c mouse embryos between 14 and 17 days old. Fibroblasts distribute throughout connective tissues during embryonic development to provide structural support and extracellular matrix essential for embryonic growth. BALB/3T12-3 cells show significant metabolic function with a specific emphasis on processing vitamin A. Research indicates that these cells have the capability to transform retinol into its metabolite anhydroretinol while also playing a role in the creation of glycoproteins. Additionally, these cells demonstrate effective glucose use for ATP production that occurs mainly through glycolytic processes.
BALB/3T12-3 cells fail to develop tumors in immunodeficient mice yet demonstrate colony formation in semi-solid media. Because it easily succumbs to herpes simplex virus (HSV) and vesicular stomatitis virus (VSV) infection this cell line serves as a common model for RNA virus research and propagation. Moreover, this cell line functions as a fibroblast model to study cell growth as well as differentiation alongside intercellular signaling processes.
MHV68 Induces Type 17 Cytokines during Virus Infection
Lung cancer leads in cancer mortality, with environmental factors like tobacco smoke causing Type 17 inflammation and aiding tumor progression. Nearly 50% of normal lung tissues show high oncogene levels, suggesting chronic Type 17 inflammation's role in lung cancer. γherpesviruses, by encoding inflammatory cytokines IL-17 and IL-6, are suspected contributors to lung tumorigenesis. Mukhopadhyay et al. used K-RasLA1 mice infected with MHV68 to simulate infection-induced inflammation, monitoring cytokine spike and immune cell changes over seven weeks to elucidate the virus's tumor-promoting mechanism via specific cytokine pathways and immune cell modifications.
BALB/3T12-3 cells were used to grow MHV68, whereas BHK-21/C-13 cells were used for viral titration. Administering 40,000 pfu of MHV68 induced maximal Orf65 mRNA expression, signaling viral replication, without severe lung inflammation or adverse effects in mice. To correlate cytokine production with viral infection, 40,000 pfu MHV68 was used on wild-type mice. RNA from lung tissues was collected daily from days 3 to 9 post-infection for RT-qPCR analysis. Day 7 mock-infected mice served as controls. Serum and lung tissues were also analyzed by ELISA for protein levels. Orf65 mRNA levels rose sharply by day 3 and declined by day 9 (Fig. 1a). Il-17 mRNA decreased by ~2.5 times after peaking on day 3, remaining low from days 7 to 9 (Fig. 1b). Il-6 mRNA levels significantly increased 40-fold on day 7 compared to controls (Fig. 1c). ELISA showed that IL-6 levels rose 1.8-fold in serum and 9.4-fold in lung lysates (Fig. 2). These results demonstrate a Type 17 cytokine response to MHV68 infection.
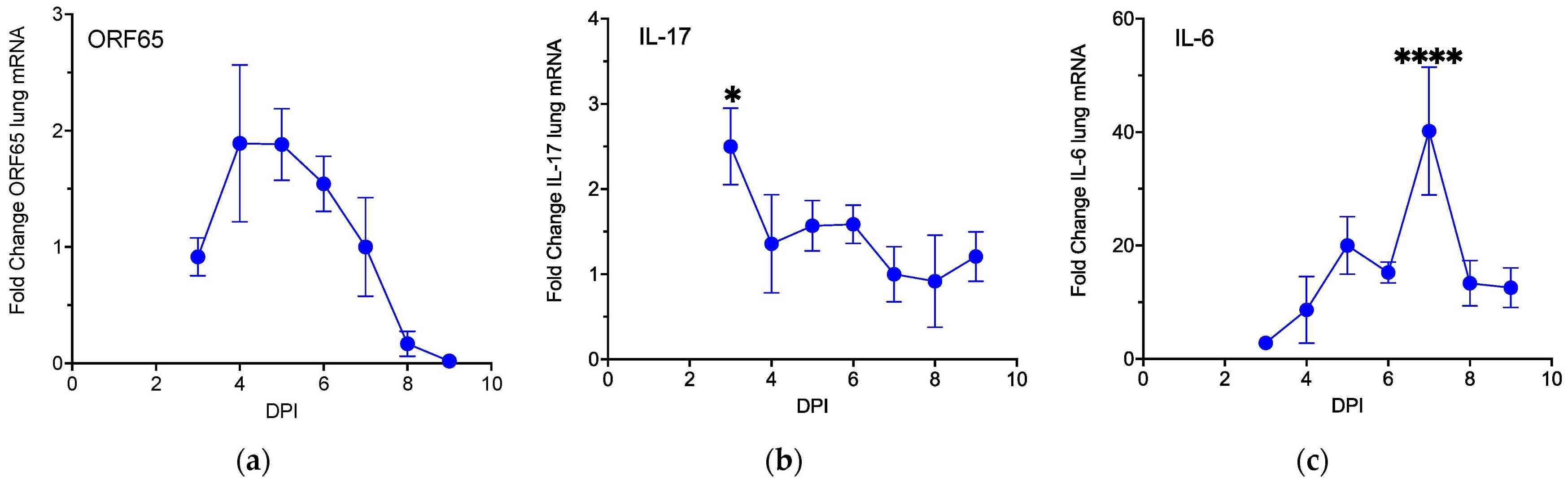 Fig. 1. MHV68 infection activates expression of Type 17 cytokines (Mukhopadhyay SS, Swan KF, et al., 2024).
Fig. 1. MHV68 infection activates expression of Type 17 cytokines (Mukhopadhyay SS, Swan KF, et al., 2024).
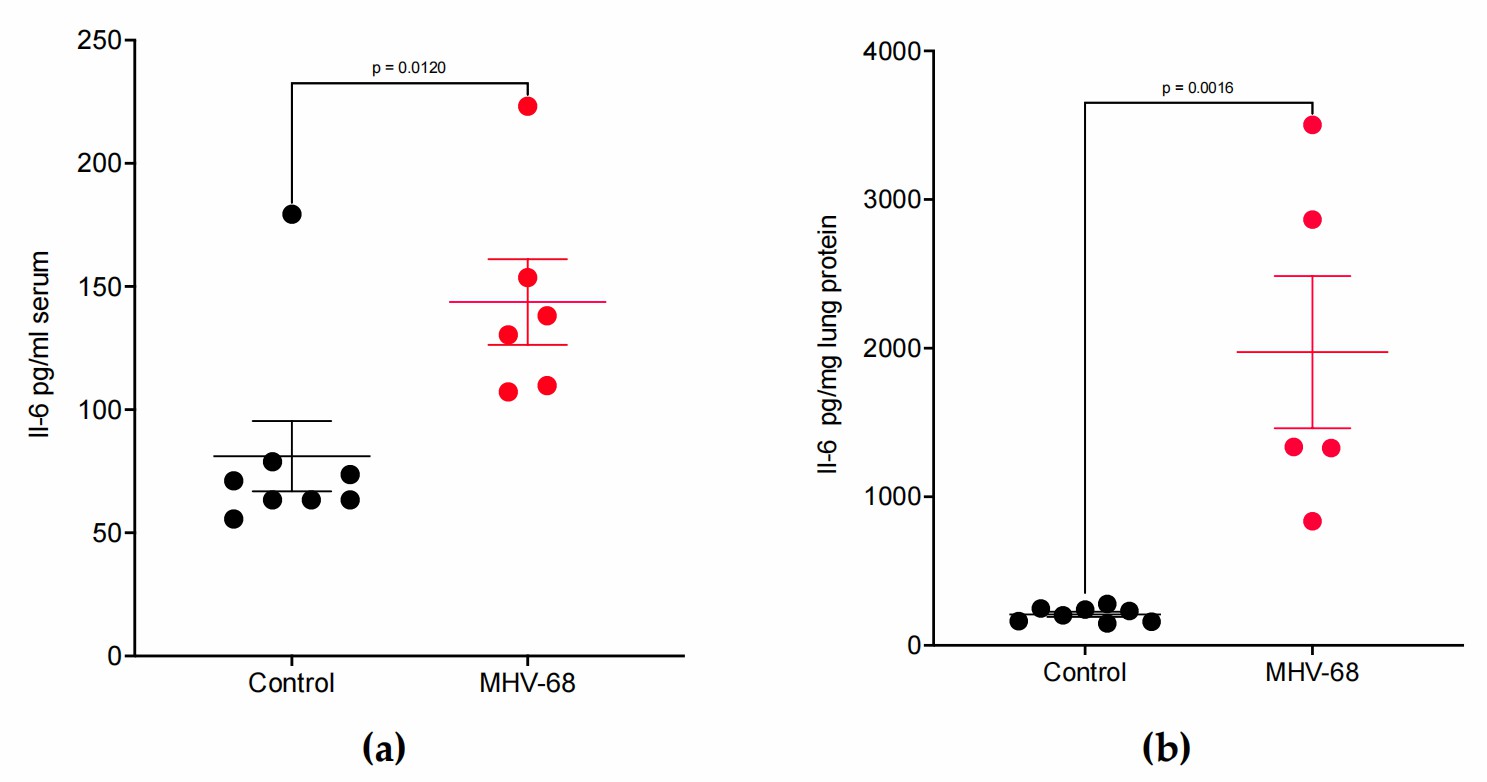 Fig. 2. MHV68 infection induces IL-6 protein expression in WT mice (Mukhopadhyay SS, Swan KF, et al., 2024).
Fig. 2. MHV68 infection induces IL-6 protein expression in WT mice (Mukhopadhyay SS, Swan KF, et al., 2024).
Virus Infection Switches the EAE Phenotype from Type A to Type B
MS, a complex autoimmune disease, shows variability in treatment responses, with many patients resistant to IFNβ. EAE, a model for MS, reveals an IFNβ-resistant subtype that lacks reliance on the NLRP3 inflammasome. Inoue et al. seeks to unravel the mechanisms governing this EAE subtype, employing an aggressive immunization protocol using high doses of heat-killed mycobacteria (Mtb) without genetic modification or additional reagents.
Type B EAE differs from type A EAE in three main ways: more inflammatory cells in the brain, extended disease duration with neuronal damage, and distinct gene and protein expression profiles. Despite these differences, type B EAE does not change the major leukocyte subpopulations. Expression patterns suggest type B EAE may prevent type A EAE development. MHV68 strains were amplified in BALB/3T12-3 cells and virus-infected cells were treated with three freeze–thaw cycles, and then supernatant was isolated. They have shown that a high-dose inoculum of MHV68 caused mice, despite type A induction, to develop NLRP3 inflammasome–independent and IFNβ-resistant EAE. MHV68 infection also increased CXCR2CD4 T cells together with mLT-expressing DCs (Fig. 3i). Furthermore, MHV68 infection converted mice with type A induction to type B EAE responses, such as reduced serum IL-1β levels and increased CXCL1 levels (Fig. 3j), allowing infected mice, despite type A EAE induction, to respond to treatments that block LTβR or CXCR2 (Fig. 3k). These results suggest strong stimulation of innate immunity triggers LTβR-CXCR2 axis to induce the type B EAE phenotype.
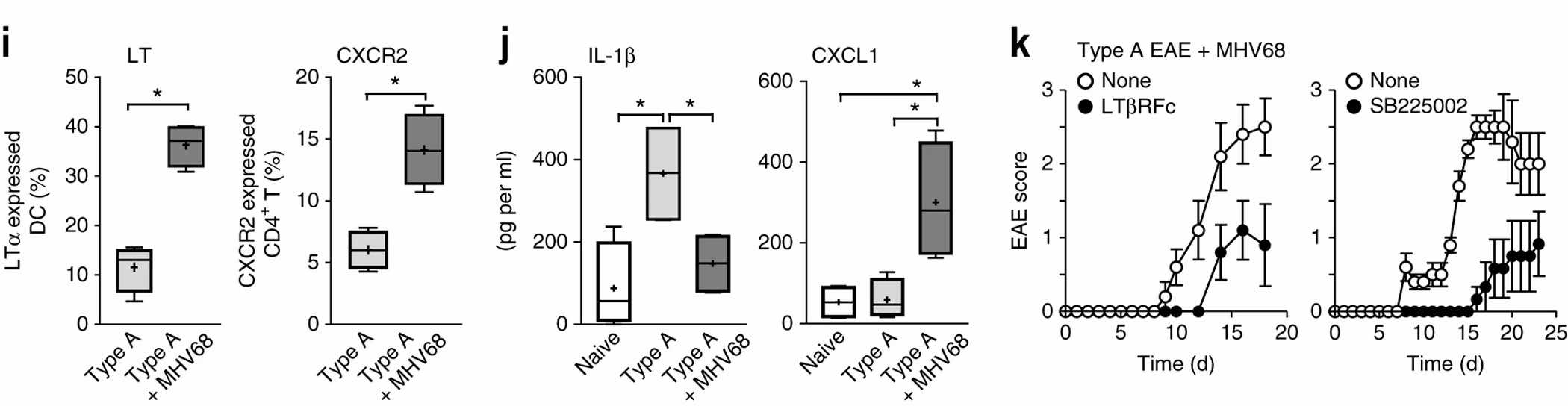 Fig. 3. Involvement of the CXCL1 and CXCR2 in development of type B EAE and type A EAE with MHV68 infection (Inoue M, Chen Ph, et al., 2016).
Fig. 3. Involvement of the CXCL1 and CXCR2 in development of type B EAE and type A EAE with MHV68 infection (Inoue M, Chen Ph, et al., 2016).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells