Human iPSC-derived microglia
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
iPSCs are pluripotent stem cells that can be differentiated into different neural cells such as neurons, astrocytes, and microglia. Microglia are primary resident immune cells of the brain that originate from the yolk sac during embryonic development and are not bone marrow-derived macrophages. They survey and clear pathogens and cellular debris in the brain and regulate immune response by releasing cytokines and chemokines. They also have strong phagocytic ability, which is important for brain homeostasis. In addition, microglia contribute to neurodevelopment by pruning synapses and fostering neuronal connections, which is important in regulating synaptic plasticity. When the brain is infected or injured, microglia become activated and secrete inflammatory factors.
iPSCs can be differentiated into microglia by adding specific cytokines and growth factors to recapitulate the development and function of human brain microglia. There are a number of methods to do so, which typically involve several steps (i.e., embryoid body formation, hematopoietic stem cell differentiation, myeloid precursor cell differentiation, and microglial differentiation) and specific growth factors (i.e., BMP4, VEGF, SCF) and cytokines (i.e., IL-34, TGF-β1, M-CSF). Specific markers for microglia, such as IBA1, CD11b, P2RY12, TREM2, and CX3CR1, can be used to identify and characterize iPSC-derived microglia.
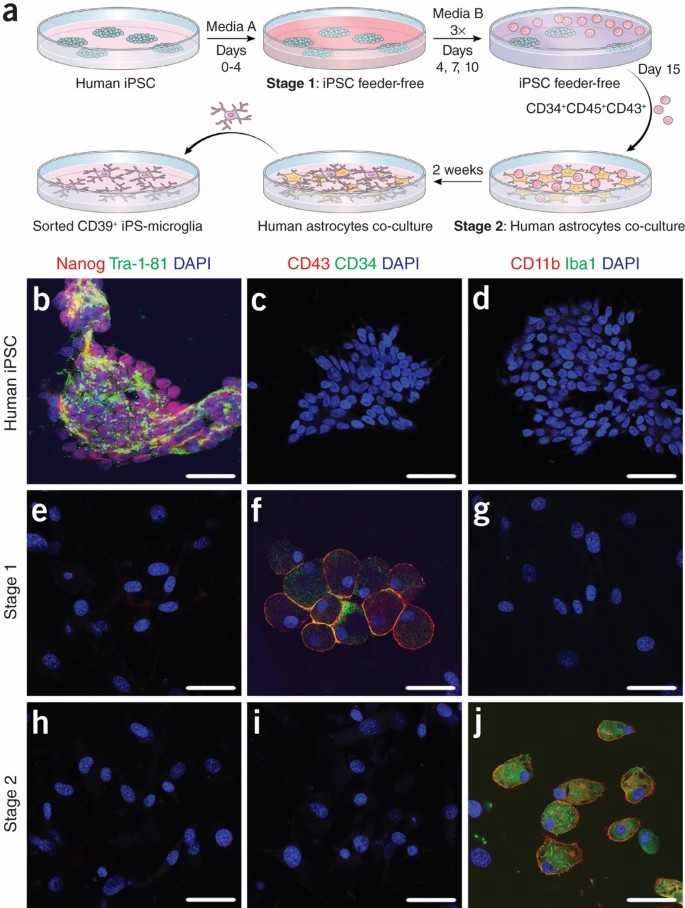 Fig. 1. Human iPSCs are differentiated into microglia-like cells (Pandya H, Shen M, et al., 2017).
Fig. 1. Human iPSCs are differentiated into microglia-like cells (Pandya H, Shen M, et al., 2017).
Human iMGLs Cluster Around Visible PM Particles upon Exposure
Air pollution, especially traffic-related particulate matter (PM), is associated with neurological diseases. PM can enter the brain directly or indirectly, potentially affecting microglia, the brain's immune cells. To explore how PM affects human microglia, Jäntti et al. used human iPSC-derived microglia-like cells (iMGLs) and exposed them to PM from diesel and compressed natural gas exhausts.
They investigated the response of human iPSC-derived microglia-like cells (iMGLs) to 24-h exposure to traffic-related particulate matter (PM) collected from urban commuter buses (Fig. 1a). EN590-PM was obtained from conventional diesel exhaust, while CNG-PM was collected from compressed natural gas exhaust using a standard method. The PM's technical properties were previously reported. After 24-h exposure, iMGLs clustered around visible EN590-PM particles, but CNG-PM was not visible and did not cause morphological changes or clustering (Fig. 1d). This clustering likely results from the organic fraction of diesel exhaust causing particles to stick together, though EN590 also contains many single particles not visible in the image.
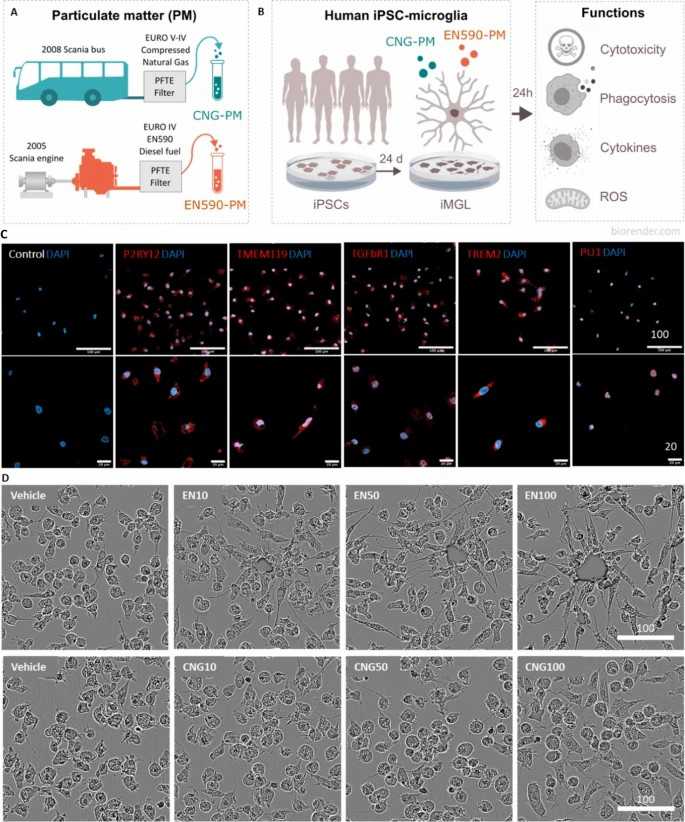 Fig. 1. Study design and characterization of human iMGLs (Jäntti H, Jonk S, et al., 2024).
Fig. 1. Study design and characterization of human iMGLs (Jäntti H, Jonk S, et al., 2024).
Iron Loading in iPSC-MG Occurs in Response to Increased Iron Concentrations but not Inflammatory Stimuli
Iron accumulation is a common feature in neurodegenerative disorders like Alzheimer's disease and Parkinson's disease, often found in microglia. Excessive iron can lead to oxidative stress and neuroinflammation, contributing to disease progression. To explore the relationship between iron and inflammation, Kenkhuis et al. treated human iPSC-derived microglia (iPSC-MG) with iron (ferric citrate) and inflammatory stimuli (IFN-γ and amyloid β).
Previous studies showed ferritin expression increased in murine microglia after inflammatory stimulation with IFN-γ and/or Aβ. They treated iPSC-MG with increasing iron concentrations, IFN-γ, Aβ (1–42), or combinations of iron with IFN-γ or Aβ. Both ferric ammonium citrate (FAC) and ferric citrate (FC) induced ferritin upregulatio, but they chose FC for further experiments due to its physiological relevance in the human brain. They measured ferritin levels and found significant increases only after FC treatment, independent of IFN-γ or Aβ (Fig. 2B and 2C). Using an iron-specific fluorescent tag, we observed that both FC and IFN-γ increased iron uptake into the cytosol (Fig. 2D and 2E). They also assessed iron-metabolism genes: FTL mRNA increased with FC treatment (Fig. 2F), while FTH1 mRNA only increased with FC, not with IFN-γ or Aβ (Fig. 2G). SLC11A2 expression decreased with FC to prevent further iron uptake but slightly increased with IFN-γ (Fig. 2H). SLC40A1 expression increased only with combined FC and IFN-γ treatment (Fig. 2I). These results indicate that FC and IFN-γ both increase cytosolic iron, but only FC induces ferritin expression and long-term iron storage.
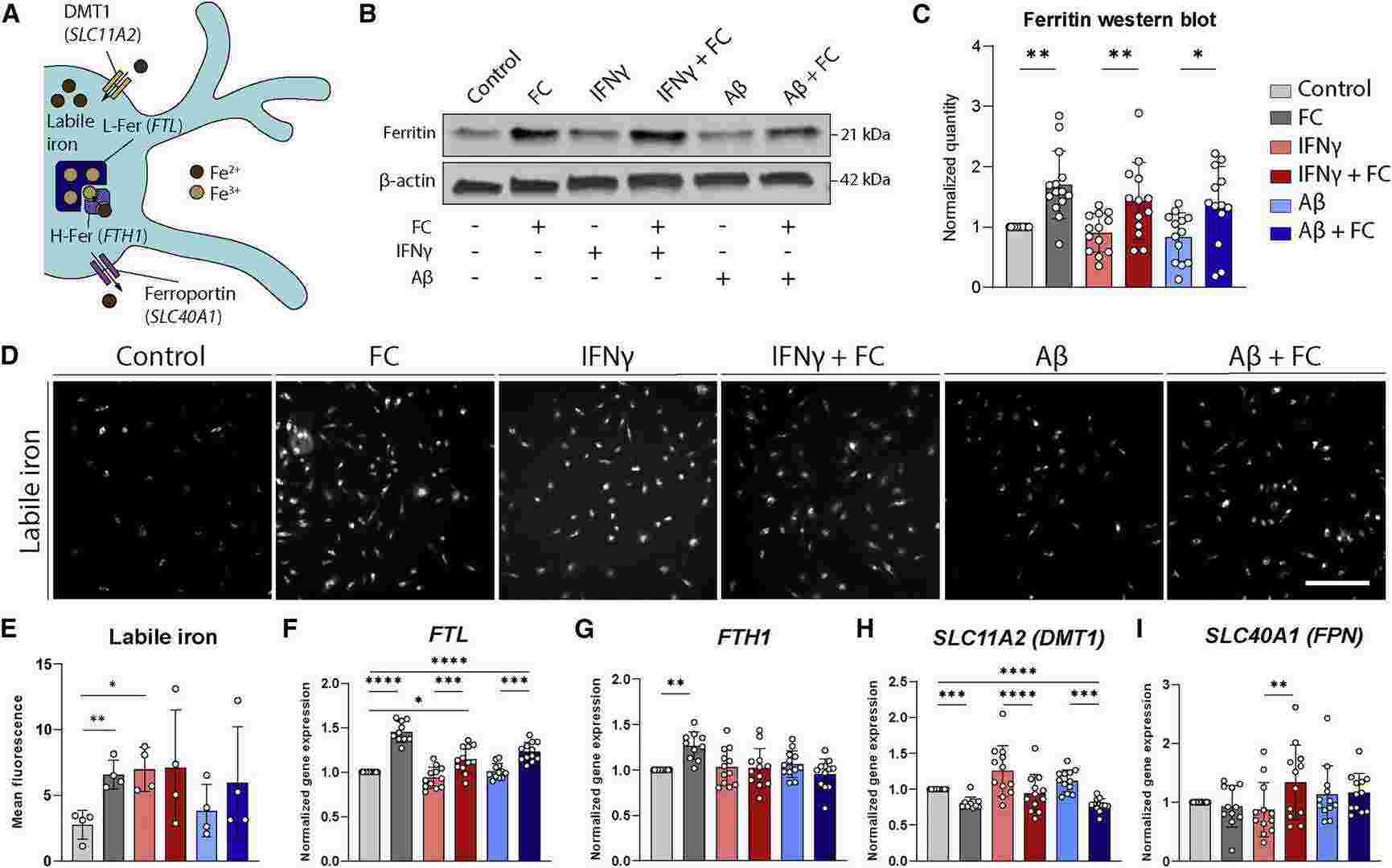 Fig. 2. Increased iron concentrations, but not inflammatory activation, lead to microglial iron loading (Kenkhuis B, Eekeren M, et al., 2022).
Fig. 2. Increased iron concentrations, but not inflammatory activation, lead to microglial iron loading (Kenkhuis B, Eekeren M, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells