Human iPSC-derived GABA neurons
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human iPSC‑derived GABAergic neurons have been derived from integration‑free iPSCs via deterministic reprogramming approaches to produce pure (>95 %) populations of inhibitory interneurons. These cells express canonical GABAergic markers (GAD1/2, VGAT/SLC32A1, DLX1/2) and, depending on the protocol, subtype‑specific proteins like parvalbumin or somatostatin. Functionally, these iPSC derived GABA neurons express spontaneous calcium transients within 48 h of plating and mature electrophysiological characteristics (resting membrane potentials < 55 mV, fast spiking firing, robust GABA evoked currents) can be measured by patch clamp or MEA platforms. The neurons also form functional synaptic networks in co culture with iPSC derived glutamatergic neurons at defined excitatory/inhibitory (E/I) ratios (e.g., 65:35) recapitulating the E/I balance of the human cortex. These networks can be interrogated for synaptic plasticity, network synchrony, and drug induced modulation.
In turn, these functional attributes support a wide range of applications. Disease modeling studies have used GABAergic iNs to model E/I dysregulation in autism spectrum disorder, schizophrenia, bipolar disorder, Huntington's disease, and other major psychiatric disorders. The cells have also been used for neurotoxicity screening and precision medicine drug discovery in the context of their human specific genetics and reproducible phenotype. Moreover, the ability to generate patient specific lines allows for personalized modeling of rare neurogenetic mutations and evaluation of therapeutic candidates in the context of a physiologically relevant inhibitory milieu.
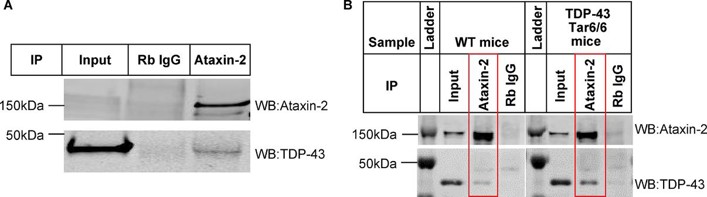
Ataxin-2 interacts with TDP-43 in both iPSC-derived GABA neurons and mouse brain tissue
Ataxin-2, containing a polyQ extension, is associated with increased ALS risk when its polyQ length is intermediate. Previous studies showed that down-regulating Ataxin-2 can mitigate TDP-43 proteinopathy in ALS models.
To identify alternative therapeutic targets, Tian's team used co-immunoprecipitation and mass spectrometry to examine the interaction between Ataxin-2 and TDP-43 and map their interactome in iPSC-derived GABA neurons. To do this, they overexpressed human TDP-43 in human iPSC-derived GABA neurons using scAAV8-Syn-TDP-43, and immunoprecipitated the lysates with IgG or an Ataxin-2 antibody. They then probed the elutes with either Ataxin-2 or TDP-43 via Western blot. They found a robust expression of both Ataxin-2 and TDP-43 in the Ataxin-2 antibody immunoprecipitants but not in the IgG control, indicating an interaction between Ataxin-2 and TDP-43 protein in iPSC neurons (Fig. 1A). They next tested this interaction between Ataxin-2 and TDP-43 in vivo, and results showed a strong interaction mouse brain tissue (Fig. 1B).
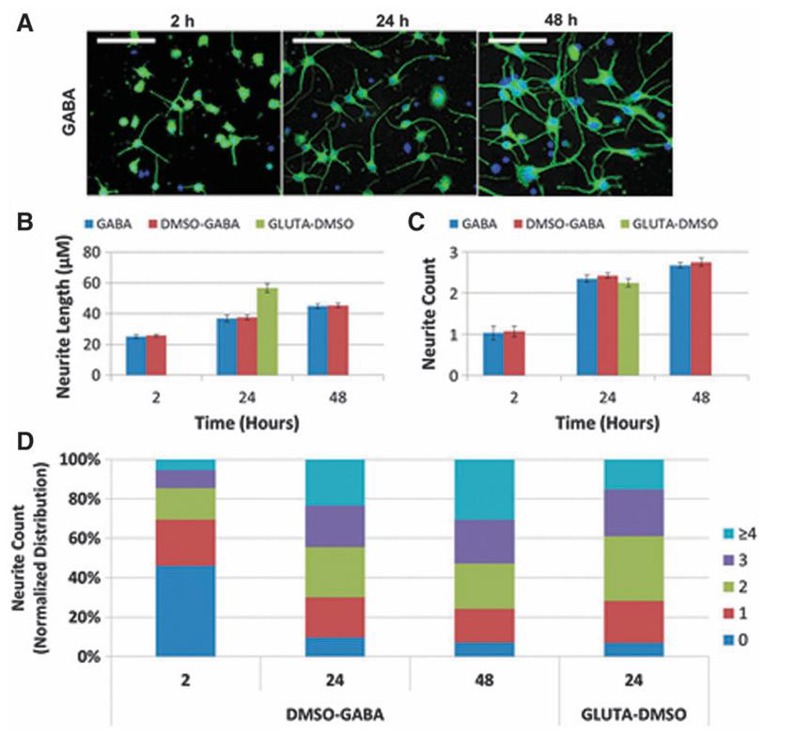
Comparative Sensitivity of Human-Induced Pluripotent Stem Cell-Derived Neuronal Subtypes to Chemically Induced Neurodegeneration
Predicting neurodegenerative potential of compounds is important for safety assessment. This study aims to compare the sensitivity of human-induced pluripotent stem cell-derived GABAergic and glutamatergic neurons to chemically induced perturbation, evaluating potential lineage-specific neurodegeneration.
To study the morphology of hiPSC-derived GABA and GLUTA neurons, an algorithm was optimized to quantify neurite outgrowth parameters, including NL, NC, normalized distribution of NC, neuron density (NpF), and cell body area. The time course of neurite outgrowth was assessed in GABA neurons at 2, 24, and 48 hours postplating (Fig. 2A). GLUTA neurons at 24 hours were also evaluated for comparison (Fig. 2). Incubation with 0.1% DMSO had no effect on any parameter. GABA neurites grew rapidly, with average lengths of 26, 38, and 45 µm at 2, 24, and 48 hours, respectively. GLUTA neurites measured 57 µm at 24 hours (Fig. 2B). GABA had more neurites per cell body but shorter processes than GLUTA at 24 hours. The largest changes in NL and NC occurred between 2 and 24 hours. The 2-hour time point was chosen to start chemical treatment, and 24 hours was selected to observe acute neurodegenerative effects.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells