Human iPSC-derived Dopaminergic Neurons
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human iPSC-derived Dopaminergic Neurons are human skin fibroblasts or blood cells which have been reprogrammed to iPSCs, then further differentiated into dopaminergic neurons. These cells have a typical neuronal morphology in culture, express dopaminergic (e.g. TH and FoxA2) and mature neuronal markers (e.g. MAP2), and exhibit calcium transient activity and action potentials, suggesting that the cells are highly mature and active.
Human iPSC-derived Dopaminergic Neurons are commonly used to model the pathological mechanism of human neurodegenerative diseases, such as Parkinson's disease. For example, it has been found that these cells recapitulate the pathological features observed in Parkinson's disease, including mitochondrial dysfunction, α-synuclein accumulation, and LRRK2 mutations. Due to their physiological similarity to patient-derived neurons, human iPSC-derived dopaminergic neurons are also used in high-throughput drug screening to test the efficacy of compounds. In the future, these cells may also be used in cell replacement therapy. For example, iPSC-derived dopaminergic neurons have been found to be viable and able to rescue behavioral deficits when transplanted into Parkinson's disease animal models.
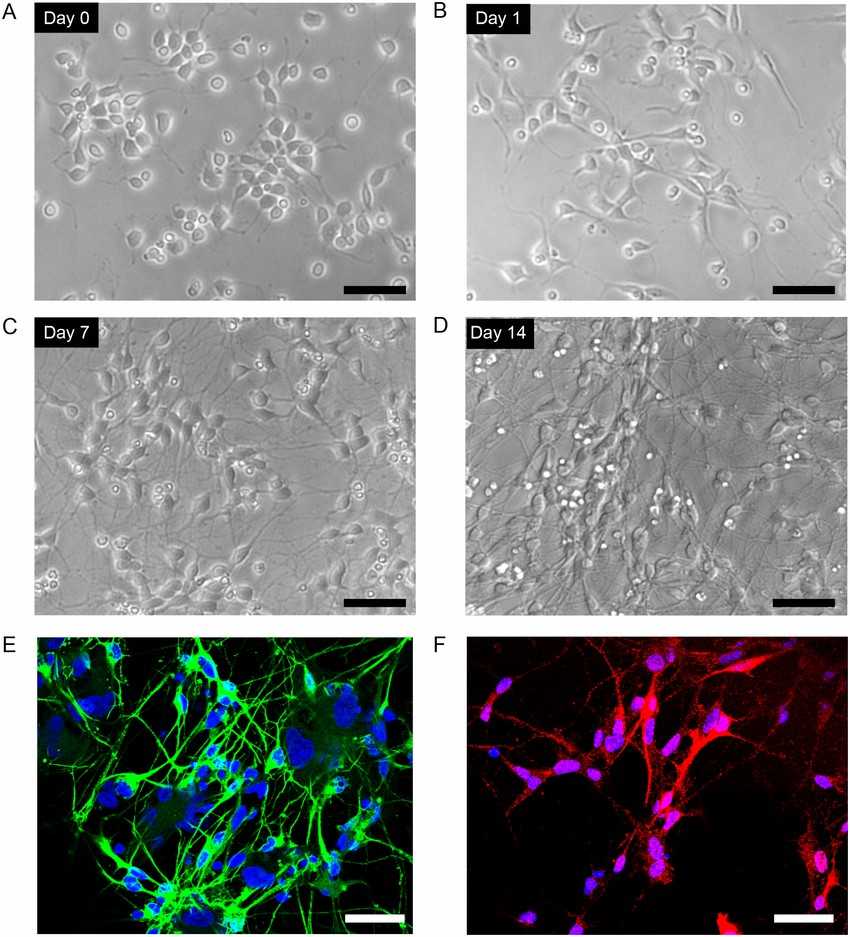 Fig. 1. Culture of human iPSC-derived dopaminergic neurons (Ito H, Uchida T, et al., 2015).
Fig. 1. Culture of human iPSC-derived dopaminergic neurons (Ito H, Uchida T, et al., 2015).
PB-II Activates the Nrf2/ARE Signaling Pathway and reduces Cellular ROS
Mechanism of PaBing-II (PB-II), a Chinese medicine for Parkinson's disease (PD) treatment, remains unclear. Wu's team employed hiPSC-derived dopaminergic neurons (DAn) and oxidative damage models to uncover the neuroprotective mechanism of PB-II.
To explore the mechanisms of PB-II protection on DAn from oxidative damage and inflammation, ROS level and inflammatory cytokines were measured in hiPSC-derived DAn after different treatments. While ROS levels remained similar between the Model and Blank Serum groups, PB-II Serum significantly reduced ROS levels under oxidative stress, indicating its antioxidant effect (Fig. 1A, B). Consistent with this, PB-II increased Nrf2 protein levels and the expression of downstream genes like NQO1, compared to no change in the Model group after H2O2 exposure (Fig. 1C–F). Additionally, PB-II increased Nrf2 nuclear translocation, similar to the Nrf2 activator artemisitene. In the ARE-luciferase reporter carrying MDA-MB-231 cells, PB-II activated ARE-mediated gene expression (Fig. 1G). ELISA results demonstrated that PB-II reduced the production of pro-inflammatory cytokines, including MCP-1, TNF-α, and IL-6, and increased anti-inflammatory IL-10 in DAn (Fig. 1H–K). In summary, the study demonstrated that PB-II protects DAn by activating the Nrf2/ARE pathway and reducing both oxidative damage and inflammation.
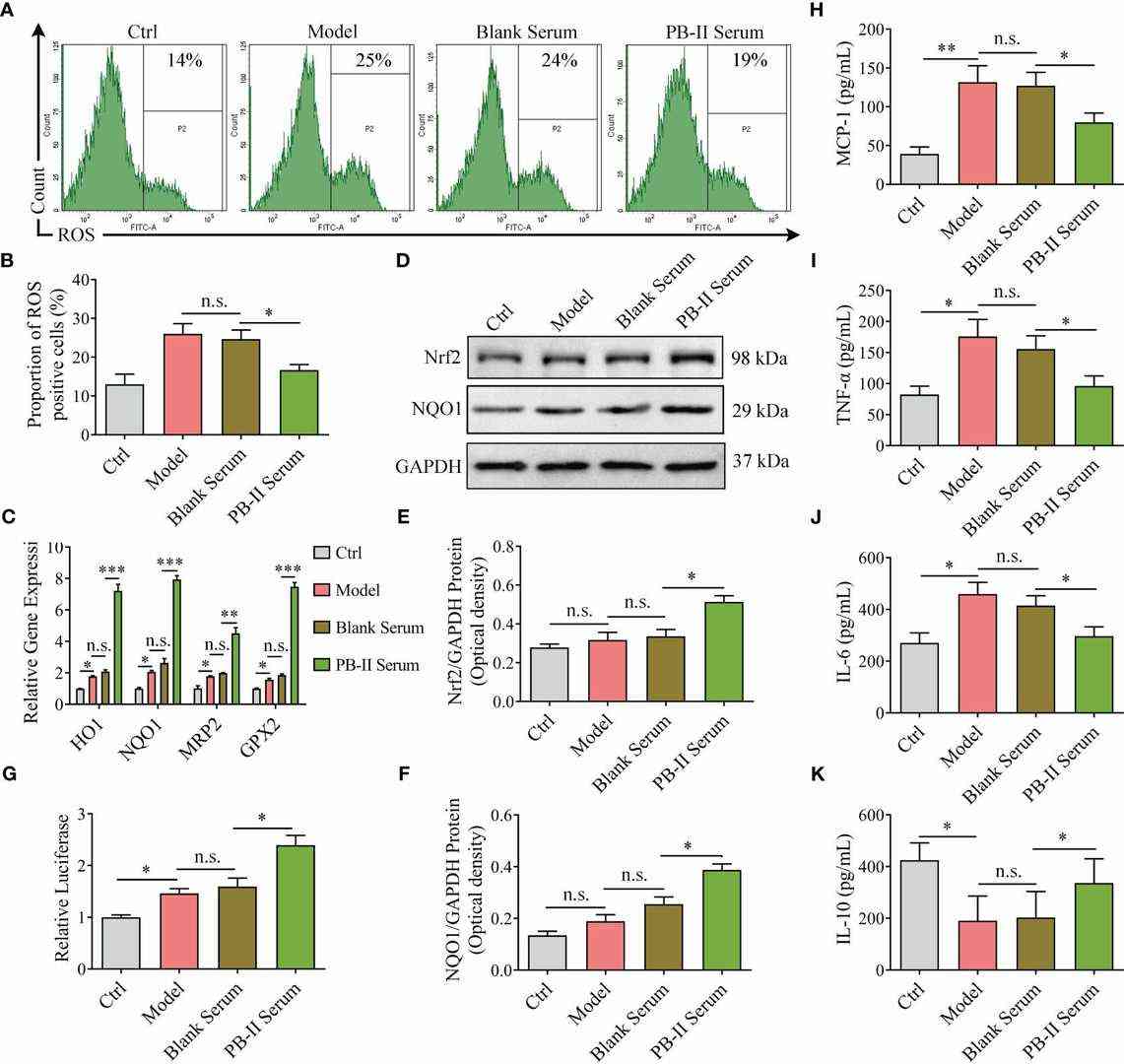 Fig. 1. Nrf2 pathway genes are activated in medicated cells and reduce ROS and inflammation (Wu S H, Rong C P, et al., 2024).
Fig. 1. Nrf2 pathway genes are activated in medicated cells and reduce ROS and inflammation (Wu S H, Rong C P, et al., 2024).
ET Protects Day 40 iDA Cultures against 6-OHDA-Induced Increase in mROS, Loss of MMP, Reduction in ATP Levels, and Loss of Dopamine Secretion
Parkinson's disease is a neurodegenerative disorder characterized by oxidative stress and mitochondrial defects in dopaminergic neurons. Ergothioneine (ET) is an endogenous antioxidant that can cross the blood-brain barrier, but it is unknown whether ET has neuroprotective effects in relevant human PD models. To establish this, Leow et al. modeled 6-OHDA-induced PD-like damage in human iPSC-derived dopaminergic neurons (iDA) and SH-SY5Y cells. Mitochondrial function, ROS levels, and protein damage were analyzed with or without ET treatment to see if ET could rescue 6-OHDA-induced neurotoxicity through OCTN1-mediated uptake.
MTT assay demonstrated that 6-OHDA induced 70–80% metabolic loss in iDA cultures (Fig. 2D). In a subset of cultures, 6-OHDA also induced neuronal degeneration, with the presence of fragmented neurons under the microscope (Fig. 3A), which is consistent with previous in vitro degeneration phenotypes. 6-OHDA increased the number of non-viable cells. iDA cultures were shown to express OCTN1, the transporter responsible for ET uptake (Fig. 2A). ET rescued iDA cultures in a concentration-dependent manner, and 1 mM ET was used in subsequent experiments (Fig. 2B). OCTN1 inhibitor VHCL was used to reduce intracellular ET levels (Fig. 2C). 6-OHDA increased the number of non-viable cells more in TH+ iDAs versus TH− non-iDAs (Fig. 3C). ET significantly reduced non-viable cells in both the TH+ and TH− populations except GM23720 non-iDAs (p = 0.233) (Fig. 3C). 6-OHDA decreased ATP levels, which was reversed by ET co-treatment (Fig. 4A). 6-OHDA also reduced dopamine secretion in iDA cultures, which was attenuated by ET co-treatment (Fig. 4B). 6-OHDA reduced mitochondrial membrane potential (MMP) and increased mitochondrial ROS levels in both cell populations, and both parameters were significantly more altered in iDAs versus non-iDAs (Fig. 4C–F). VHCL abolished ET-mediated rescue.
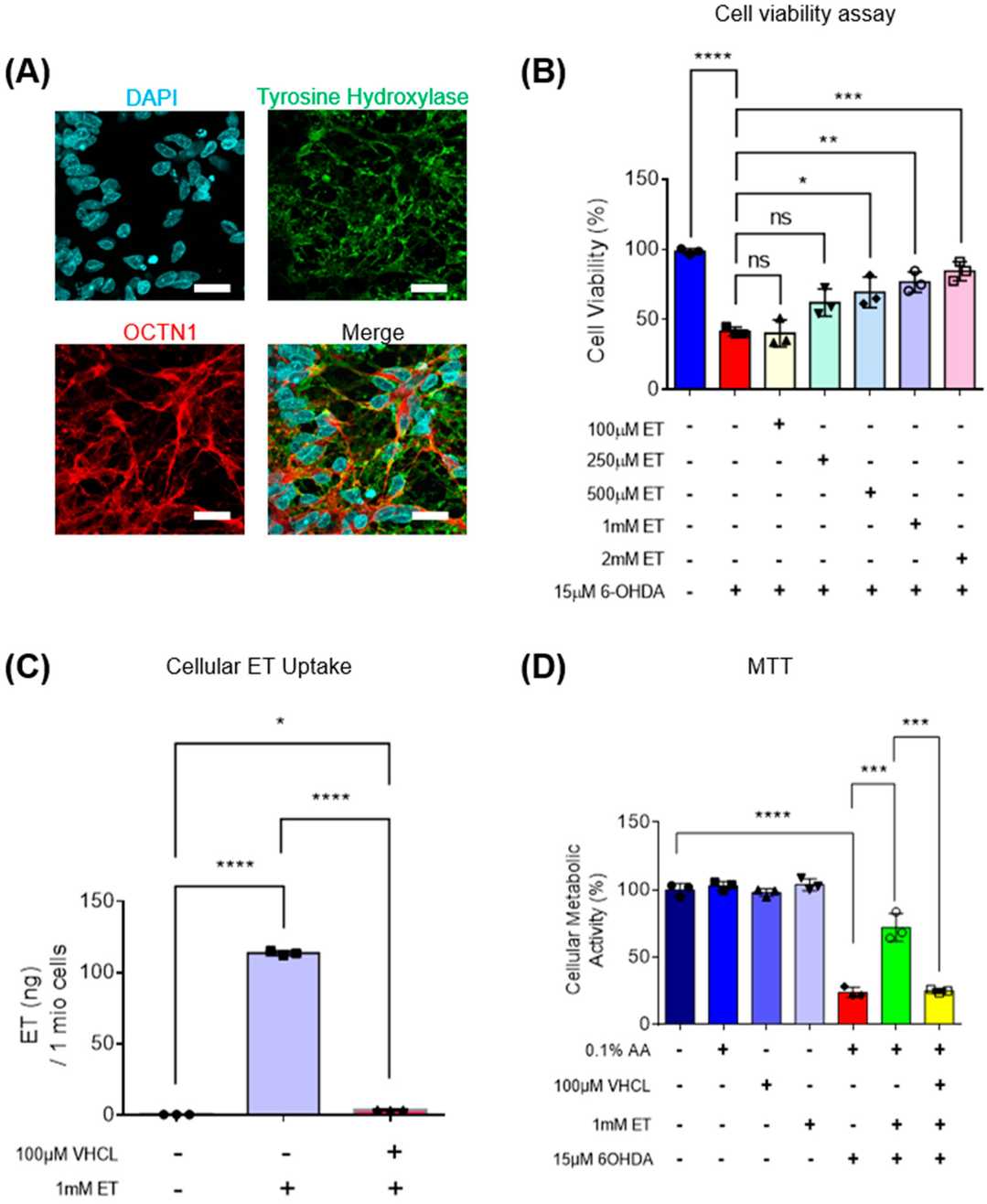 Fig. 2. Intracellular uptake of ET, mediated through OCTN1 transporters, protects iDA cultures against 6-OHDA-induced cell death in a concentration-dependent manner (Leow DM-K, Cheah IK-M, et al., 2024).
Fig. 2. Intracellular uptake of ET, mediated through OCTN1 transporters, protects iDA cultures against 6-OHDA-induced cell death in a concentration-dependent manner (Leow DM-K, Cheah IK-M, et al., 2024).
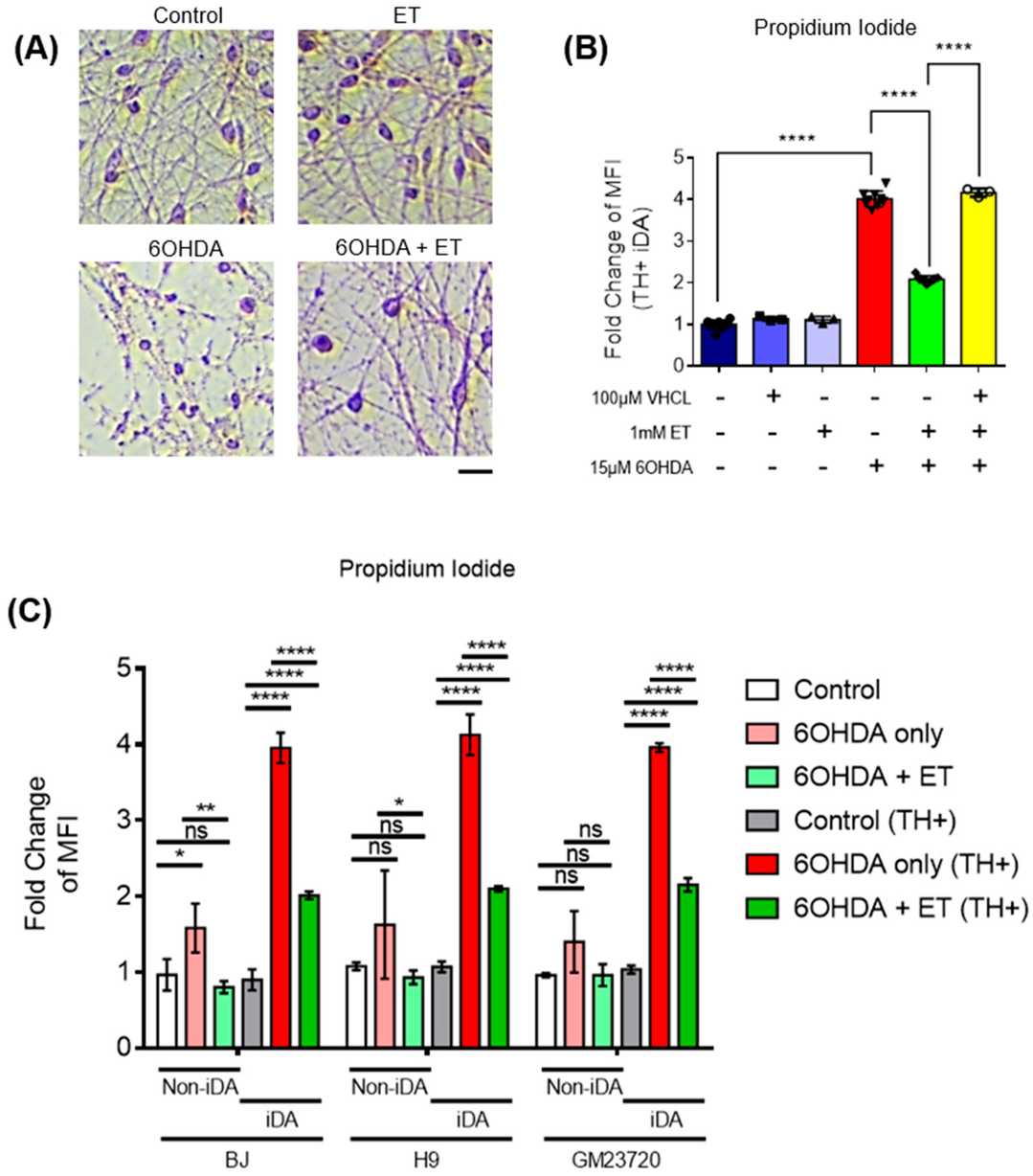 Fig. 3. ET-mediated protection of 6-OHDA-treated iDAs and non-iDAs in culture (Leow DM-K, Cheah IK-M, et al., 2024).
Fig. 3. ET-mediated protection of 6-OHDA-treated iDAs and non-iDAs in culture (Leow DM-K, Cheah IK-M, et al., 2024).
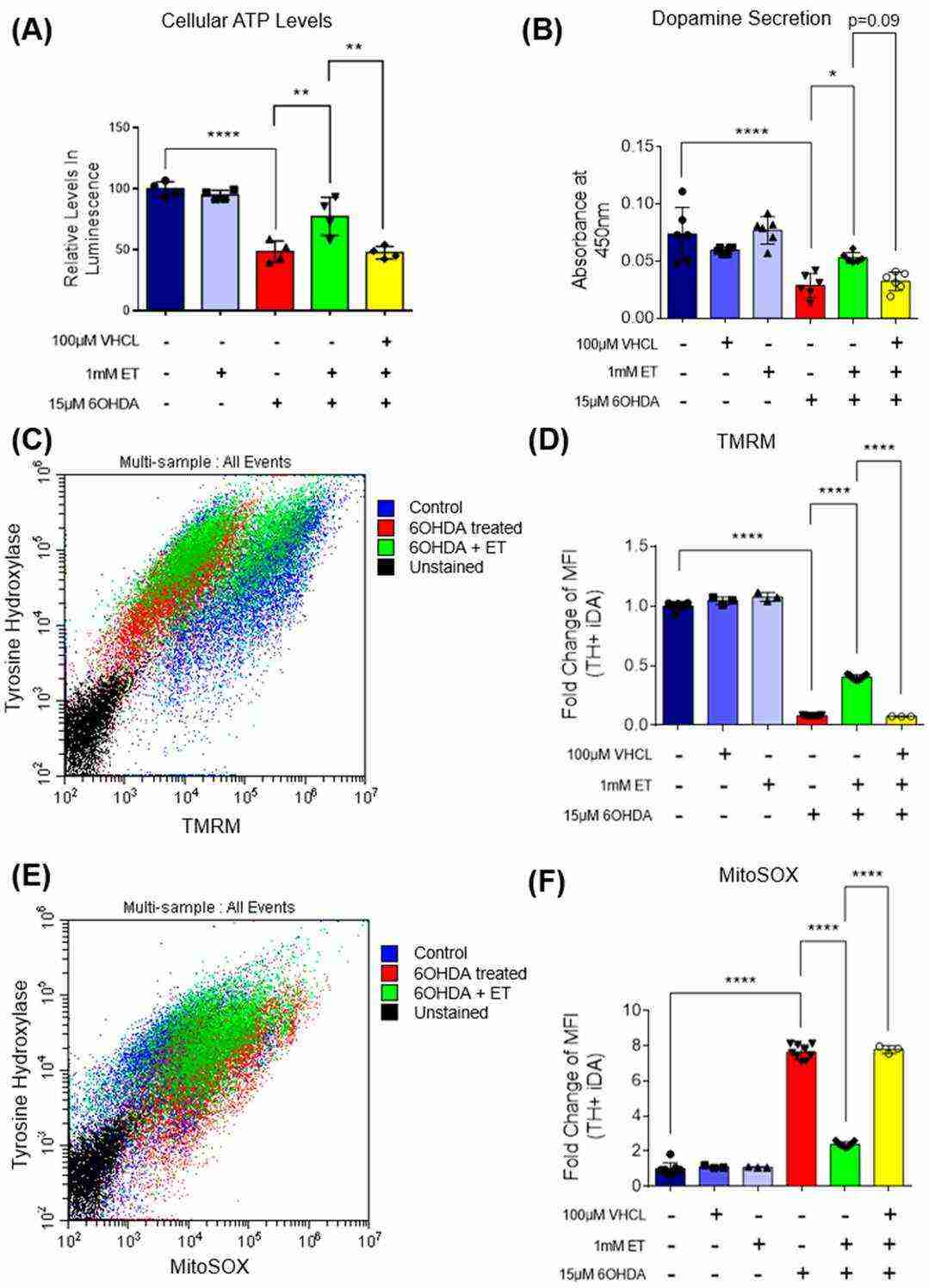 Fig. 4. Effect of ET on intracellular ATP levels and dopamine secretion in 6-OHDA-treated iDA cultures; TH+ Day 40 iDAs were also analysed for mitochondrial ROS and mitochondrial membrane potential levels (Leow DM-K, Cheah IK-M, et al., 2024).
Fig. 4. Effect of ET on intracellular ATP levels and dopamine secretion in 6-OHDA-treated iDA cultures; TH+ Day 40 iDAs were also analysed for mitochondrial ROS and mitochondrial membrane potential levels (Leow DM-K, Cheah IK-M, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells