Human iPSC-derived Astrocytes
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human iPSC-derived Astrocytes are cultured cells that are differentiated from induced pluripotent stem cells (iPSCs). The iPSCs are usually generated from fibroblasts collected from healthy donors. These astrocytes expand as a monolayer during in vitro culture while showing polarized morphologies and express glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100b) as markers. During cultivation, they display the characteristic morphology of astrocytes, featuring branched processes similar to protoplasmic astrocytes.
Human iPSC-derived Astrocytes are an important tool for neuroscience research and disease modeling. They are commonly used to model astrocyte dysfunction in neurodegenerative diseases, neurodevelopmental disorders, and neuroinflammatory diseases. For instance, these cells can be used to study the role of astrocytes in neurodegenerative diseases such as Alzheimer's and Parkinson's, as well as the pathological mechanisms involved in neurodevelopmental disorders like leukodystrophies. In addition, they can be used for drug screening and toxicity testing to assess the potential neurological effects of new drugs.
Increased Clearance of Dead Cells in Co-Cultures of Neurons and Aβ-Exposed Astrocytes
Astrocytes are vital for brain homeostasis and synaptic function but are also linked to Alzheimer's disease (AD) pathology. The previous research showed that astrocytes take up large amounts of aggregated amyloid-beta (Aβ) but store rather than degrade it, causing cellular stress. However, the role of pathological astrocytes in AD-related synaptic dysfunction is unclear. Konstantinidis et al. investigated how intracellular Aβ deposits in astrocytes (human iPSC-derived astrocytes) affect their interaction with neurons, focusing on neuronal function and viability.
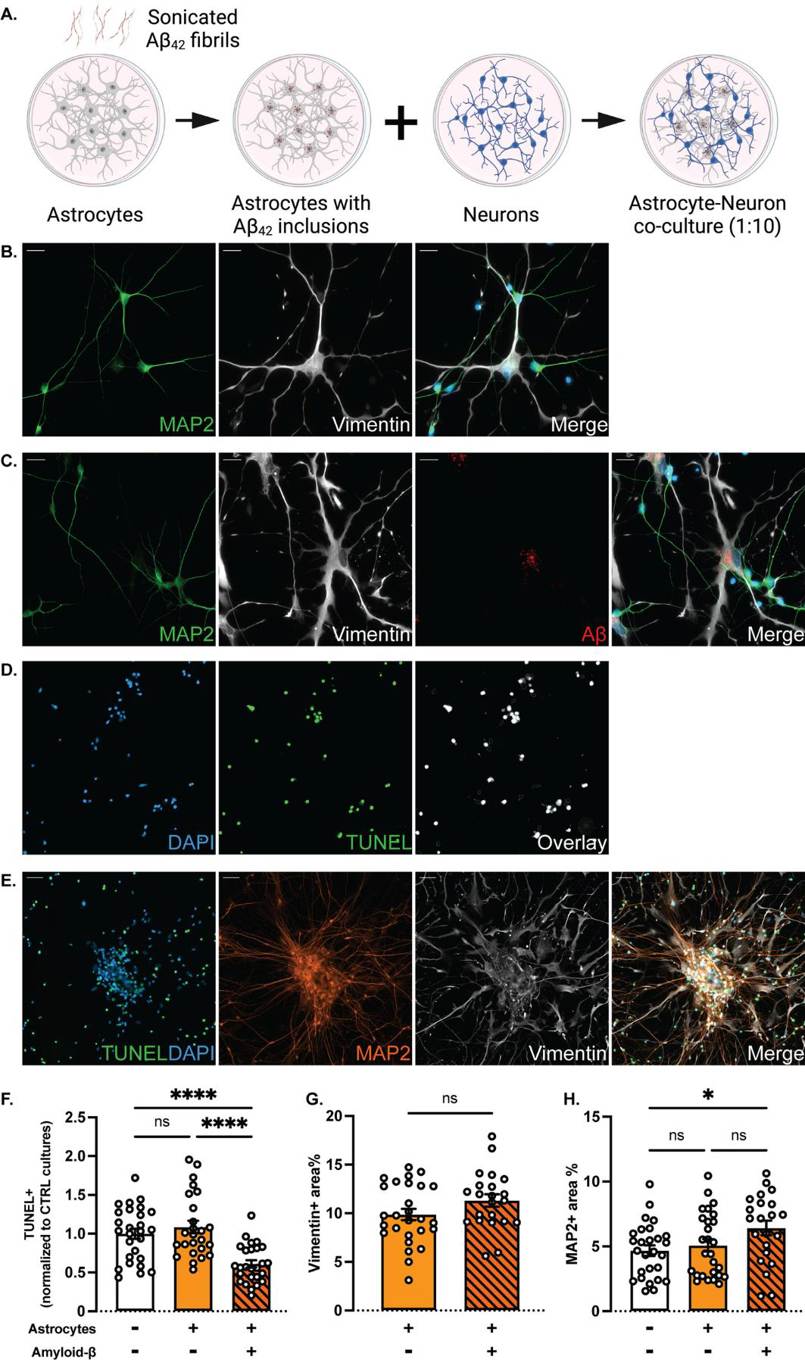
To study the effects of Aβ pathology on astrocyte-neuron interactions, they established co-culture systems of astrocytes (with or without Aβ inclusions) and healthy neurons. Astrocytes exposed to Aβ were seeded onto neuronal cultures 7 days after their plating at a 1:10 ratio (Fig. 1A). The astrocytes maintained Aβ inclusions after reseeding. After 7–10 days of co-culture, immunocytochemistry revealed that both cell types remained mature and well-integrated into the network (Fig. 1B, C). The close contacts and apparent gap junctions between astrocytes and neurons are indicative of direct communication (Fig. 1C). TUNEL assays show fewer apoptotic neurons in co-cultures with Aβ astrocytes compared to control cultures (Fig. 1D, F). Aβ-exposed astrocytes more effectively cleared cell corpses (Fig. 1E). Quantification of vimentin and MAP2 staining revealed no change in the astrocyte area, but a significant increase in MAP2-positive area in Aβ co-cultures, suggesting an increased neuronal survival in Aβ co-cultures (Fig. 1G, H).
Human iPSC-Derived Astrocyte Progenitors Engraft the Mouse Brain and Differentiate into Astrocytes
Increasing evidence for a direct contribution of astrocytes to neuroinflammatory and neurodegenerative processes causing Alzheimer's disease comes from molecular and functional studies in rodent models. However, these models may not fully recapitulate human disease.
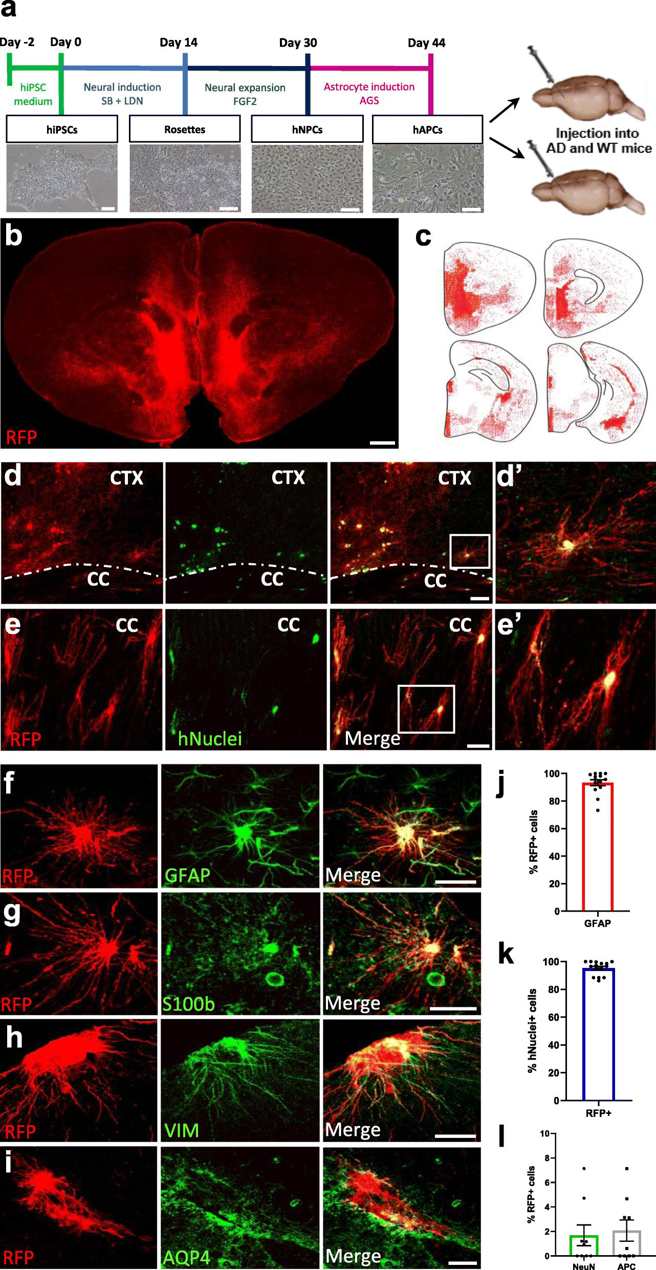
To study human astrocytes in the mouse brain, Preman et al. transplanted hiPSC-derived astrocyte progenitors into neonatal mouse brains. They differentiated hiPSCs into astrocyte progenitor cells (hAPCs) in vitro (Fig. 2a) and grafted td-Tomato-expressing hAPCs into newborn mice. They used APP/PS1-21 mice crossed with NOD-SCID mice to create AD mice or wild-type (WT) littermates for grafting. We transplanted hiPSC lines from AD patients with APOE E4/E4 alleles and corrected APOE E3/E3 lines. Five months later, immunofluorescence showed human cell engraftment throughout the forebrain (Fig. 2b). Human cells were identified by td-Tomato (RFP) and human nuclear antigen (hNuclei). RFP+ cells were seen infiltrating the cortex, corpus callosum and other subcortical structures (Fig. 2c-e). Engraftment varied in degree across cell lines. Some engrafts show robust engraftment of transplanted cells (Fig. 2b, c). At this time point, these RFP+ cells robustly expressed astrocyte markers GFAP, S100b, Vimentin and Aquaporin-4 (Fig. 2f-i) with Aquaporin-4 localized to astrocytic end-feet (Fig. 2i). Quantification revealed 93% of RFP+ cells expressed GFAP (Fig. 2j) and 95% of hNuclei+ cells co-expressed RFP (Fig. 2k). Together this confirmed that engrafted cells were differentiated to the astrocyte fate. There was minimal co-expression of neuronal or oligodendroglial markers in RFP+ cells (<3%, Fig. 2l).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells