Feline Mesenchymal Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
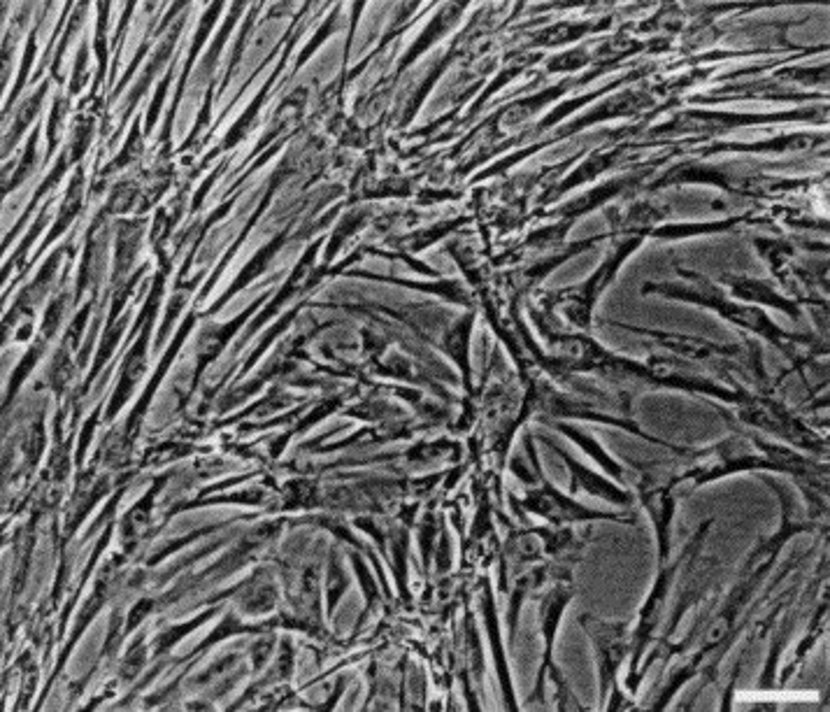
Feline Mesenchymal Stem Cells (fMSCs) are multipotent stromal cells isolated from various tissues in cats, such as bone marrow, adipose tissue, and umbilical cord. These cells exhibit the typical characteristics of mesenchymal stem cells, including plastic adherence, fibroblast-like morphology, and the ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages under appropriate conditions. fMSCs also display immunomodulatory properties and secrete trophic factors that contribute to tissue repair and regeneration.
fMSCs are commonly used in research to study feline stem cell biology, tissue engineering, and regenerative therapies. They have potential applications in veterinary medicine for treating conditions such as osteoarthritis, kidney disease, and inflammatory disorders in cats. fMSCs are also used as a comparative model for human MSC research to understand species-specific differences in stem cell behavior. Additionally, due to their self-renewal capacity and low immunogenicity, fMSCs are being investigated for allogeneic transplantation in veterinary medicine.
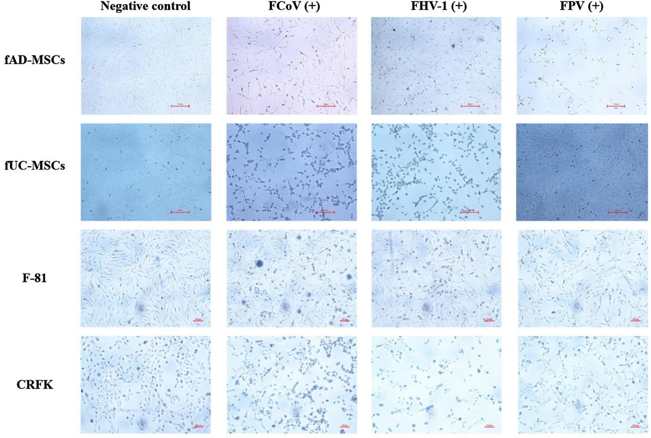
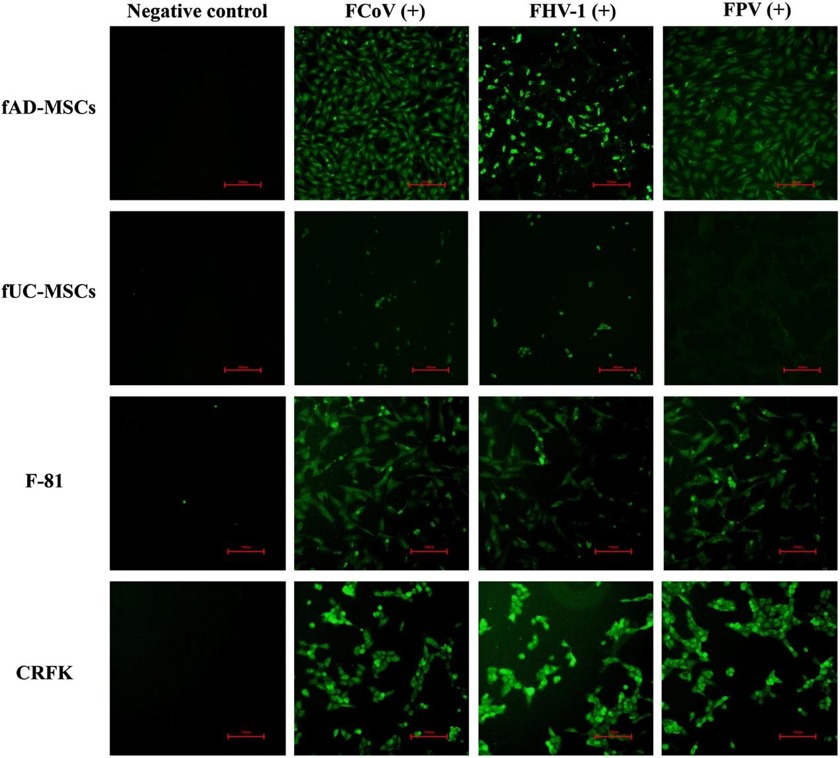
FCoV, FHV-1, and FPV Exhibit Sustained Proliferation and Replication within Fad-Mscs
Feline mesenchymal stem cells (fMSCs) have strong differentiation potential and have been widely used in feline immune-related disease studies. However, whether fMSCs are vulnerable to virus infection remains unknown. Ma et al. evaluated the susceptibility of feline adipose-derived (fAD-MSCs) and umbilical cord-derived mesenchymal stem cells (fUC-MSCs) to the common feline virus feline coronavirus (FCoV), feline herpesvirus type 1 (FHV-1), and feline panleukopenia virus (FPV).
FCoV, FPV, and FHV-1 were inoculated in fAD-MSCs and fUC-MSCs, with CRFK and F-81 as controls. FCoV and FHV-1 showed typical cytopathic effects (CPEs) in each cell line. After 48 h postinfection (hpi), the cells were observed under a microscope and presented grape-like changes, and the virus suspension was subjected to freeze‒thaw, filtration, and reinoculation up to the 10th generation with stable CPEs (Fig. 1a). FPV had no significant CPE in fUC-MSCs (Fig. 1b). The titers of the fifth-generation virus suspension were determined by the Reed-Muench method. The strains were named FCoV-YBYJ-2024, FPV-YBYJ-2024, and FHV-1-YBYJ-2024. They used IFA to analyze the FCoV, FHV-1, and FPV staining in fAD-MSCs, fUC-MSCs, CRFK, and F-81. After 48 h, the cells infected with FCoV and FHV-1 showed strong immunofluorescence signals, and cell morphology was well defined in fAD-MSCs, CRFK, and F-81 (Fig. 2a, c). FPV-infected fAD-MSCs, CRFK, and F-81 induced fluorescence, but fUC-MSCs did not, suggesting that FPV could not proliferate in fUC-MSCs (Fig. 2b).
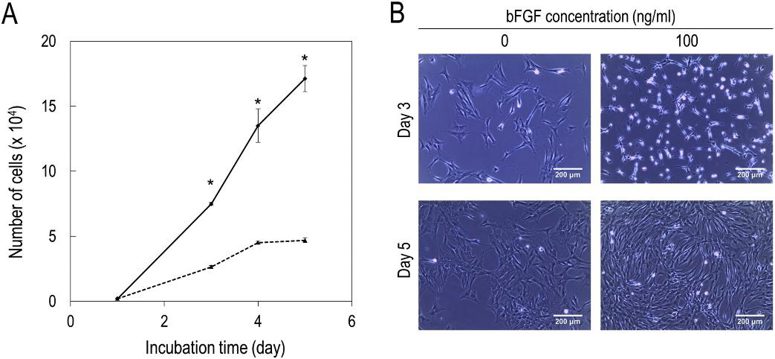
Effect of bFGF on the Proliferation and Morphology of Feline MSC
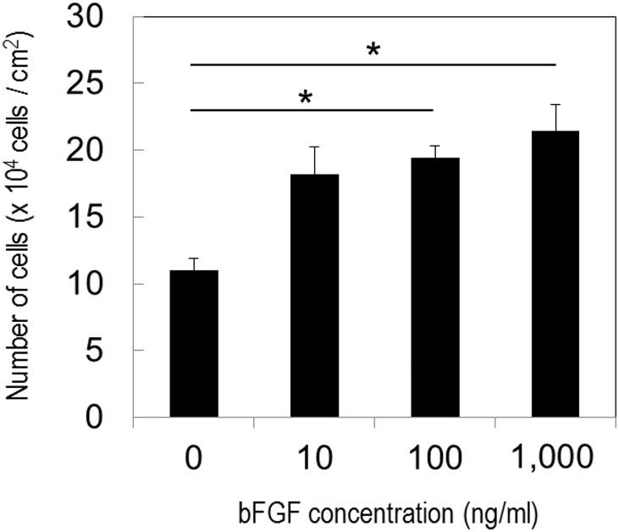
Fujimoto et al. experiment demonstrated that bFGF could enhance the proliferative potential and change the cell shape of cat adipose-derived MSCs. Cat subcutaneous adipose tissue-derived MSCs were cultured in the presence or absence of bFGF.
Fig. 3 depicts an increase in the number of MSCs and alteration in the cell shape by bFGF. The cells maintained in the absence of bFGF were shown to increase in number with the passage. After a culture period of 5 days, the cells grew in size and transformed into a flat structure. On the other hand, MSCs in the presence of 100 ng/ml bFGF proliferated more and assumed various small elongated or round shapes. In contrast, they had flattened shapes in the absence of bFGF. This shape change in MSCs was maintained for about 4 days, and their shapes partly recovered to those of MSCs without bFGF by day 5 (Fig. 3). Fig. 4 illustrates that the proliferative potential of MSCs was dependent on the concentration of bFGF. The number of MSCs was significantly higher at 100 and 1000 ng/ml bFGF than in the absence of bFGF. However, at 1000 ng/ml bFGF, the growth of MSCs was unstable. It was shown to be retarded initially, and dead cells were present, but it accelerated after 3 days.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells