JURL-MK1
Cat.No.: CSC-C0540
Species: Homo sapiens (Human)
Source: Blood; Peripheral Blood
Morphology: round cells growing singly or in small clusters in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD4 -, CD13 +, CD15 +, CD33 +, CD34 +, CD41 +, CD42 +, CD71 +, CD116 +, CD117 +, HLA-DR +
Viruses: PCR: EBV -, HBV -, HCV -, HIV -, HTLV-I/II -, SMRV -
Researchers developed the JURL-MK1 cell line from the blood of a chronic myeloid leukemia patient. Chronic myeloid leukemia represents a myeloproliferative disorder where the bone marrow becomes infiltrated with abnormal leukemia cells. These cells display typical leukemia cell morphology through their irregular nuclear structures and abnormal cytoplasmic buildup.
The JURL-MK1 cell line serves as an essential model for apoptosis research. For example, research indicates that imatinib triggers significant apoptosis in JURL-MK1 cells through early-stage activation of caspase-3 and DNA fragmentation. The JURL-MK1 cells show additional apoptosis-related molecular mechanisms including p53 protein and Glycophorin A expression which are both essential for apoptosis regulation. Researchers also utilized the JURL-MK1 cell line to investigate drug sensitivity and resistance mechanisms. For example, AICAR demonstrates its potential in resistance research by inhibiting JURL-MK1 cell metabolism which causes cell death while simultaneously killing drug-resistant cells with specific mutations. Moreover, the JURL-MK1 cells maintain their viability to a certain extent when exposed to low-temperature conditions. Studies measuring ATP content and other markers of viability after freeze-thaw cycles demonstrate how JURL-MK1 cells maintain their survival functions in cold environments.
Activated T Cell-Conditioned Medium Strongly Counteracts Imatinib-Induced Apoptosis of JURL-MK1 Cells, and IFNγ Neutralization Strongly Inhibits This Anti-Apoptotic Effect
Tyrosine kinase inhibitors (TKIs) have revolutionized chronic myeloid leukemia (CML) therapy, yet residual disease persists due to factors within the microenvironment. In particular, TKI treatment increases interferon γ (IFNγ) production, which appears to counteract imatinib-induced apoptosis in CML cells. Ujvari et al. aim to elucidate how IFNγ mediates a STAT1-dependent anti-apoptotic effect, including its role in upregulating survival proteins in CML stem/progenitor cells.
To study the role of IFNγ in the effect of activated T cell secretome on imatinib-treated CML cells, they conducted IFNγ neutralization experiments on the JURL-MK1 CML cell line using conditioned medium from T cells isolated from healthy donor PBMCs (Fig. 1). Imatinib induced high apoptosis rates in JURL-MK1 cells with non-activated T cell medium, but this effect was strongly counteracted by medium from CD3/CD28 bead-activated T cells. Importantly, a neutralizing anti-IFNγ antibody largely reversed the anti-apoptotic effect of activated T cell medium, though not completely. However, simultaneous neutralization of IFNγ and GM-CSF nearly abolished the anti-apoptotic effect of activated T cell medium on JURL-MK1 cells (Fig. 1b), indicating that the anti-apoptotic activity of activated T cell secretome is mainly mediated by IFNγ, with a minor contribution from GM-CSF.
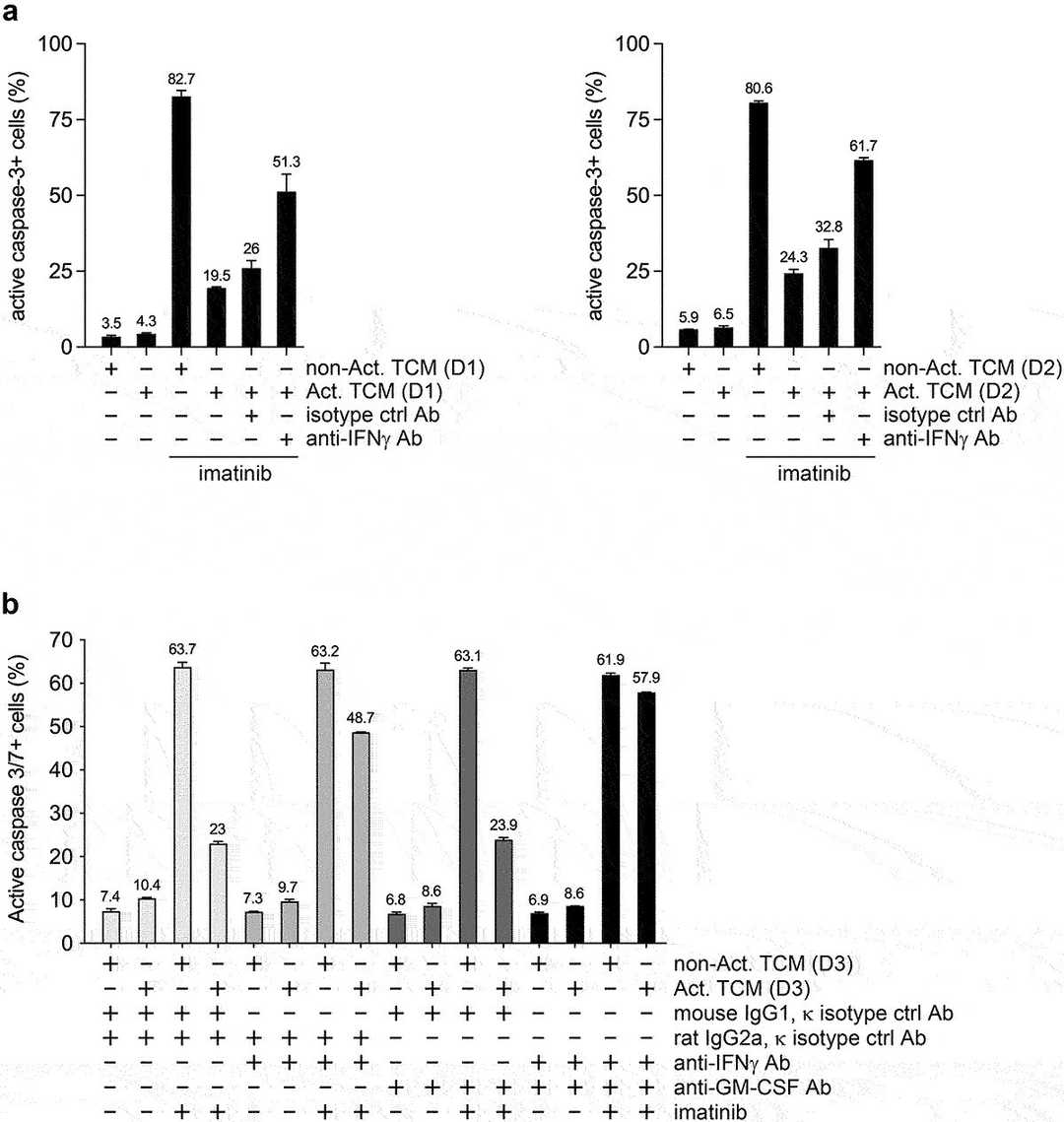 Fig. 1. IFNγ neutralization markedly counteracts the anti-apoptotic effect of activated T cell-conditioned medium on imatinib-treated JURL-MK1 cells (Ujvari D, Malyukova A, et al., 2020).
Fig. 1. IFNγ neutralization markedly counteracts the anti-apoptotic effect of activated T cell-conditioned medium on imatinib-treated JURL-MK1 cells (Ujvari D, Malyukova A, et al., 2020).
Montelukast induces Apoptosis in K562 and JURL-MK1 Cells
Chronic myeloid leukemia (CML) is characterized by the Bcr-Abl oncoprotein, and TKIs have limitations in eradicating leukemic stem cells (LSCs) due to aberrant pathways like leukotriene signaling. CysLT1R, a receptor in this pathway, is overexpressed in cancers and targeted by montelukast. Zovko et al. previously showed that montelukast reduces CML cell growth. Here, they aim to further investigate the role of CysLT1R in CML cell survival and the mechanisms by which montelukast induces apoptosis in CML cells.
Treatment with montelukast and imatinib, caused cytosol localization of cytochrome c (Fig. 2A). Release of cytochrome c leads to activation of caspases and cleavage of the caspase-3 substrate PARP-1. Montelukast treatment resulted in PARP-1 protein cleavage in the K562 cells. Ratio of cleaved vs. full length PARP-1 increased in a dose dependent manner (Fig. 2B). Furthermore, they observed a dose-dependent increase in proapoptotic Bax protein signal in K562 cells upon montelukast treatment (Fig. 2B). The growth of JURL-MK1 cells was significantly inhibited by montelukast, and when combined with imatinib the receptor antagonist induced further growth inhibition (Fig. 2C). PARP-1 protein was also cleaved in JURL-MK-1 cells treated with montelukast (Fig. 2D). Montelukast (4 μM) also induced significant caspase-3 activation (Fig. 3A and B). Imatinib, which was used as a positive control for apoptosis induction, activated caspase-3 but when used in combination with 4 μM montelukast, caspase-3 was further activated. Moreover, the caspase inhibitor Z-VAD was able to partially inhibit montelukast-induced cell death (Fig. 3C).
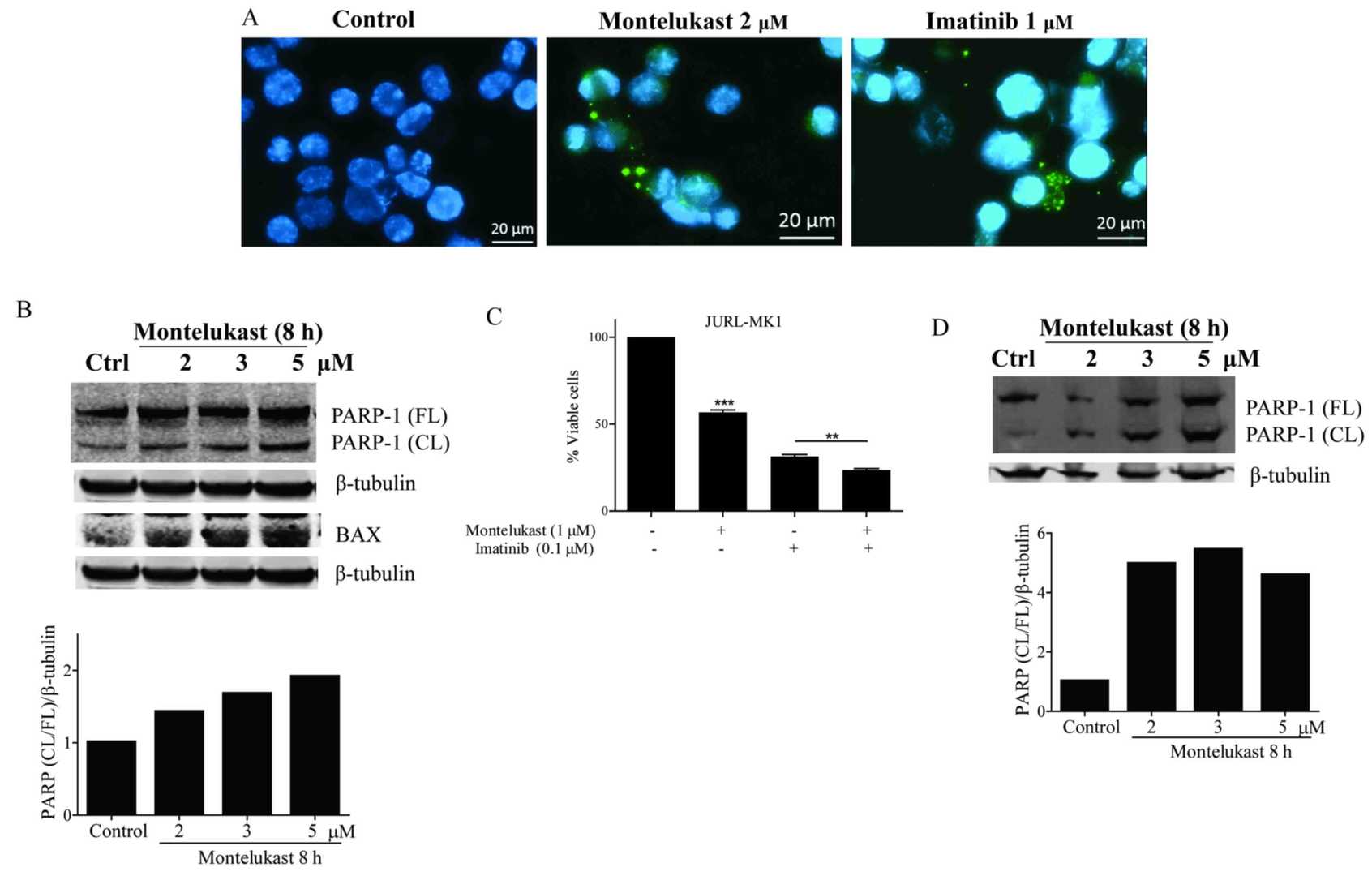 Fig. 2. Montelukast induces apoptosis in K562 and JURL-MK1 cells (Zovko A, Yektaei-Karin E, et al., 2018).
Fig. 2. Montelukast induces apoptosis in K562 and JURL-MK1 cells (Zovko A, Yektaei-Karin E, et al., 2018).
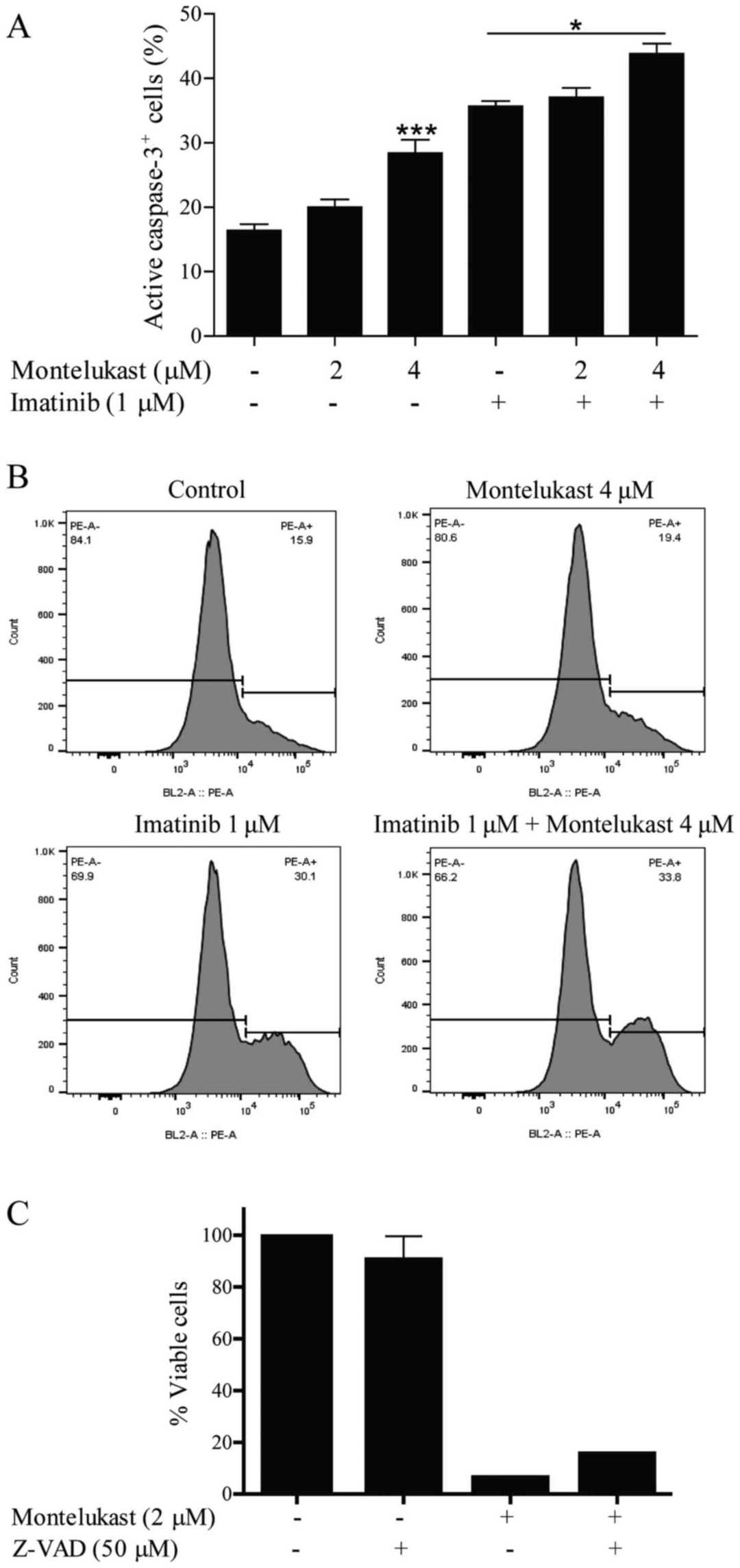 Fig. 3. Caspase-3 activation in the K562 cell line (Zovko A, Yektaei-Karin E, et al., 2018).
Fig. 3. Caspase-3 activation in the K562 cell line (Zovko A, Yektaei-Karin E, et al., 2018).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells