Human Adipose-Derived Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human adipose-derived stem cells (hASCs), also known as adipose-derived mesenchymal stem cells (ASCs), are mesenchymal stem cells that are isolated from adipose tissue. Adipose tissue is most commonly harvested by liposuction from subcutaneous fat deposits in the abdomen, thighs, or buttocks, though it can also be obtained from visceral fat deposits or resected fat pads in an abdominal surgical procedure. They are located in the stromal vascular fraction of adipose tissue, in a perivascular niche. The cells have a spindle-shaped, fibroblast-like morphology when cultured, with a large nucleus and nucleoli.
They can differentiate into adipogenic, osteogenic, chondrogenic, myogenic, and neural-like cells. hASCs have strong immunomodulatory capabilities and produce trophic factors along with paracrine effects that include anti-inflammatory action and angiogenic properties. These features have contributed to research into their use in a variety of diseases, including autoimmune diseases, tissue ischemia, and others. They are often used in tissue-engineered constructs, 3D bioprinting, and disease modeling, especially in the context of metabolic disease, such as obesity and diabetes. They have also been investigated as a research tool in cancer research, including their ability to home to tumors for potential use in drug delivery.
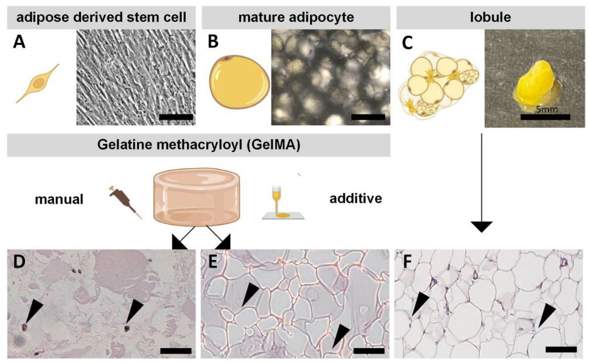
Bioprinting of 3D Adipose Tissue Models Using a GelMA-Bioink with Human Mature Adipocytes or Human Adipose-Derived Stem Cells
Adipose tissue is of high importance in numerous diseases, requiring disease relevant in vitro models. Available models lack physiological relevance and therefore limit their research potential. Albrecht et al. developed bioprinted 3D adipose tissue models using GelMA-based bioinks containing human mature adipocytes (MA) or adipose-derived stem cells (ASCs).
ASCs are the adipocyte precursors and can give rise to adipogenic lineages. Mature adipocytes (MA) on the other hand are terminally differentiated. ASCs are adherent spindle-shaped cells (Fig. 1A), while MAs are large, round, non-adherent cells (Fig. 1B). Both cell types were isolated from adipose tissue (Fig. 1C). The cells were encapsulated in GelMA, and then manually and additively fabricated. ASC-laden models were induced to undergo adipogenic differentiation for two weeks, while the MA-containing models were cultured for one week to match the lobule conditions. Histological staining (Fig. 1D–F) showed that native adipose tissue has a high cell density (white lipid vacuoles), and a lack of ECM (red eosin staining). The GelMA models show reduced lipid vacuoles and increased matrix due to the lower cell density. The MA-laden models most closely recapitulated native tissue due to the presence of lipid vacuoles. DiffASCs also formed lipid droplets, however, they were smaller (Fig. 1D). Morphologically, MAs better recapitulated the native tissue than diffASCs. The density of cells in the models cannot be increased arbitrarily, as GelMA stability is impaired.
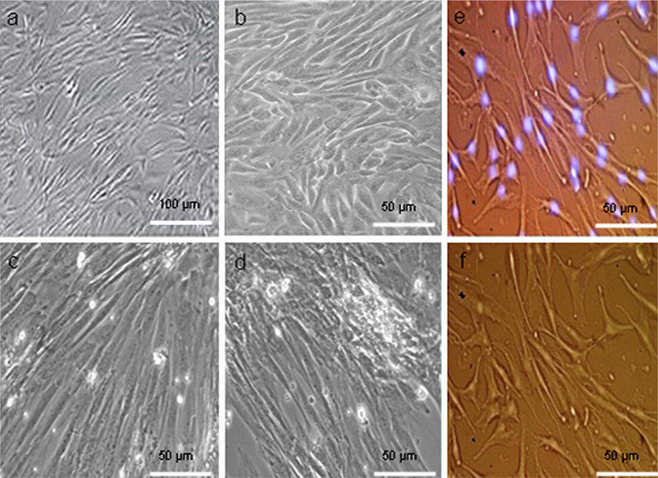
METTL3 Promoted Osteogenesis of hASCs Induced by NELL-1 In Vitro
Adipose-derived stem cells (hASCs) have the potential to be induced to differentiate into osteoblasts. NEL-like 1 protein (NELL-1) promotes osteogenesis. However, the role of METTL3-mediated lncRNA m6A modification in NELL-1-induced osteogenesis of hASCs remains unclear. Song et al. explored how METTL3 regulated lncRNA m6A methylation to control hASC osteogenic differentiation under NELL-1 induction conditions.
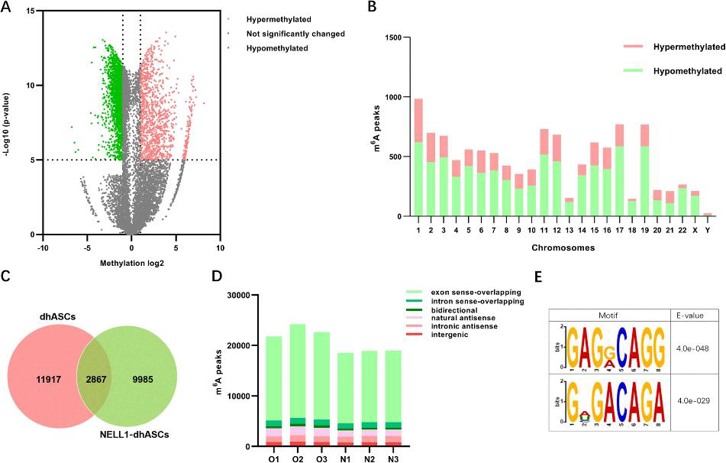
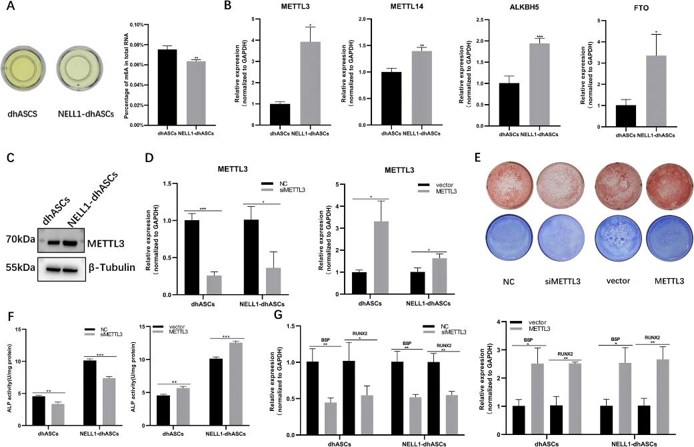
MeRIP-seq revealed extensive lncRNA m6A modifications during hASC osteogenic differentiation, with exon sense-overlapping peaks being most abundant. Differential methylation analysis identified 11,462 peaks (3,379 hyper-/8,082 hypomethylated) enriched on chromosome 1, predominantly containing RRACH motifs, suggesting m6A's regulatory role in NELL-1-induced osteogenesis (Fig. 2). The quantitative m6A assay showed higher total RNA m6A modification levels in dhASCs than those in NELL1-dhASCs, indicating a decreasing trend of m6A with osteogenesis (Fig. 3a). NELL1-dhASCs displayed higher expression of m6A-related methyltransferases and demethylases (METTL3/14, ALKBH5/FTO) and METTL3 protein (Fig. 3b and c). Functional assays of METTL3 overexpression/knockdown cells demonstrated that METTL3 regulated NELL-1-induced osteogenic differentiation. METTL3 knockdown impaired mineralization (ARS/ALP staining, Fig. 3e), ALP activity (Fig. 3f), and expression of osteogenic-related genes (RUNX2/BSP, Fig. 3g). METTL3 overexpression promoted mineralization (Fig. 3e), ALP activity (Fig. 3f), and osteogenic-related genes (Fig. 3h). These results confirmed that METTL3 is required for NELL-1-induced osteogenic differentiation.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells