Sarcoma Cells
- Background
- Applications
- Scientific Data
Sarcoma is a rare and diverse group of cancers that arise from the connective tissues in the body, which include bones, cartilage, fat, muscle, and blood vessels. Sarcoma cells originate from primitive mesenchymal stem cells that have the potential to differentiate into a variety of connective tissue cell types. Due to this multipotent nature, sarcomas can be categorized into over 50 subtypes, each with distinct genetic and histological features. The World Health Organization (WHO) classifies sarcomas based on the type of tissue from which they arise, such as osteosarcoma (bone), chondrosarcoma (cartilage), liposarcoma (fat), rhabdomyosarcoma (muscle), and angiosarcoma (blood vessels), among others.
Sarcoma cells exhibit a range of cellular characteristics that contribute to their aggressive nature. They often display anaplastic features, such as a high nuclear-to-cytoplasmic ratio, increased mitotic activity, and a tendency to form abnormal mitotic figures. The cells may also demonstrate a wide spectrum of differentiation, from well-differentiated cells that closely resemble their tissue of origin to highly undifferentiated cells that are challenging to classify.
Types of Sarcoma Cells
- Soft tissue sarcoma cells. Soft tissue sarcomas arise from the soft tissues of the body, such as muscle, fat, fibrous tissues, and blood vessels. Soft tissue sarcoma cells originate from mesenchymal stem cells that have the potential to differentiate into various soft tissue cell types. The diversity of cell types gives rise to the numerous subtypes of soft tissue sarcomas, each with its own histological appearance and genetic profile. Soft tissue sarcoma cells are characterized by their spindle-shaped appearance under the microscope and a propensity for rapid growth and invasion. They can be aggressive, with a high likelihood of metastasis to the lungs and other organs.
- Bone sarcoma cells. Bone sarcomas are cancers that originate from the bone or its associated connective tissues. Bone sarcoma cells typically arise from primitive bone-forming cells or mesenchymal cells that have the potential to differentiate into bone or cartilage. The most common types of bone sarcomas include osteosarcoma, Ewing sarcoma, and chondrosarcoma. Bone sarcoma cells differ from soft tissue sarcoma cells in their ability to produce bone or cartilage matrix. These tumors often present with localized pain, swelling, and occasionally pathologic fractures. They are known for their high grade and rapid growth, with a significant risk of metastasis, particularly to the lungs.
Drug Discovery and Development
Sarcoma cells serve as valuable models for evaluating the efficacy of new therapeutic agents. Researchers often use sarcoma cell lines and patient-derived xenografts (PDXs) to assess the effectiveness of potential drugs. Investigating the synergistic effects of combining different drugs can lead to more effective treatment protocols. Studies have explored the combination of chemotherapy agents with targeted therapies to enhance effectiveness against specific sarcoma subtypes.
Biomarker Discovery
Identifying biomarkers associated with sarcoma cells can facilitate early diagnosis and prognostication. Genetic profiling of sarcomas has revealed mutations and expression patterns that correlate with treatment responses. For example, the presence of specific translocations in Ewing sarcoma cells can guide targeted therapy decisions. Certain biomarkers can predict responses to specific treatments. For example, the expression of the CDK4/6 inhibitor sensitivity can help tailor therapies for liposarcoma patients.
Immunotherapy Research
The immune landscape of sarcomas is complex, and understanding sarcoma cells' interactions with the immune system is crucial for developing effective immunotherapeutic strategies. Researching the potential of checkpoint inhibitors and CAR-T cell therapies in targeting sarcoma cells, aiming to enhance the immune response against these tumors. Techniques such as CAR-T cell therapy, which involves engineering a patient's T cells to target specific tumor antigens, are being explored in sarcoma treatment. Research is ongoing to identify suitable targets on sarcoma cells for this approach.
Cell Stiffness Serves as a Label-Free Biomarker for Musculoskeletal Sarcoma Cells
Cell stiffness has previously been proposed to serve as a label-free biomarker for cancer detection, and refers to a cell's ability to deform in response to external stress. Five different sarcoma cell lines: chondrosarcoma, osteosarcoma, Ewing sarcoma, fibrosarcoma, and rhabdomyosarcoma were used. For each cell type, 270 atomic force microscopy (AFM) measurements (n = 3 biological replicates, 30 cells/replicate, 3 repetitions/cell) were conducted. Cancer cells were significantly less stiff (more elastic) than their corresponding non-malignant counterparts (osteosarcoma cells-osteoblasts: p < 0.001, Ewing sarcoma cells-MSC: p < 0.001, fibrosarcoma cells-fibroblasts: p < 0.001, rhabdomyosarcoma cells-SKMC: p < 0.001) except chondrosarcoma cells and chondrocytes, where the trend was the opposite (p < 0.001, Fig. 1). Absolute values were thereby reduced by 69% for the osteoblast-osteosarcoma group (median: 0.842 kPa to 0.256 kPa), 31% for the MSC-Ewing sarcoma (median: 0.381 kPa to 0.260 kPa), 70% for the fibroblasts-fibrosarcoma (median: 1.008 kPa to 0.315 kPa) and 36% for the SKMC-rhabdomyosarcoma group (median: 0.418 kPa to 0.267 kPa), while for the chondrocyte-chondrosarcoma group a notable increase in stiffness (i.e., a lower elastic profile) was observed (median: 0.239 kPa to 0.414 kPa corresponding to a 73% increase).
Since cytoskeletal remodeling may be a major contributor to the observed differences in cell stiffness between cancer cells and their healthy counterparts, the cytoskeletal structure was examined by F-actin and β-tubulin labeling. The results presented in Fig. 2A-J show that in comparison to all cancer cells (Fig. 2B, D, F, H, J), healthy cells show denser, better-aligned F-actin with longer stress fibers. Chondrocytes (Fig. 2A) showed a similar actin pattern, however at a lower density. For the stiffer healthy cells (Fig. 2C, E, G, I) the actin filaments are dispersed throughout the cell body, with actin bundles aligned along the long axis of the cell with well-defined stress fibers. Actin filaments in the softer cancer cells, in contrast, are less organized, and F-actin bundles are oriented randomly with short segments, forming a tangled network (Fig. 2D, F, H, J). All cancer cells showed a predominant cortical F-actin structure, with the majority of filament lying in the cell's periphery (Fig. 2B, D, F, H, J).
All of the cancer cell lines- healthy controls duo had a strong perinuclear presence of β-tubulin, forming a tortuous microtubular structure with longitudinally and obliquely concentrated crossed filaments, with a decreasing tendency toward the cytoplasm's periphery. This effect was especially noticeable in cancer cells (Fig. 2D, F, H, J), where the β-tubulin presence was reduced in F-actin-enriched areas of the cell periphery. The chondrosarcoma cells (Fig. 2B) exhibited a similar β-tubulin distribution to their healthy counterparts (Fig. 2A), with long, rich, well-defined, and elongated microtubule networks, a feature shared by the rest of the healthy cells (Fig. 2E, G, I).
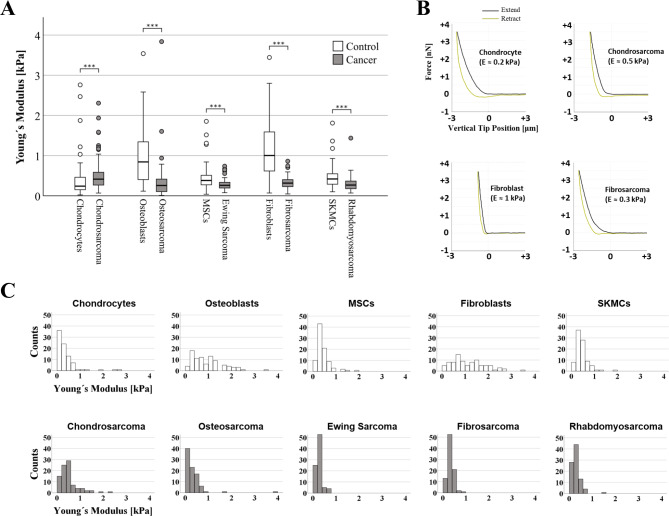 Fig. 1 (A)
Box plots (medians, first and third quartiles, minimum, maximum) of the cellular stiffness (kPa) for each cell
line are displayed. (B) Representative force-distance curves for various cell types. (C) Images showing
histogram distribution of elastic modulus of all cell types. (Daniel C, et al, 2023)
Fig. 1 (A)
Box plots (medians, first and third quartiles, minimum, maximum) of the cellular stiffness (kPa) for each cell
line are displayed. (B) Representative force-distance curves for various cell types. (C) Images showing
histogram distribution of elastic modulus of all cell types. (Daniel C, et al, 2023)
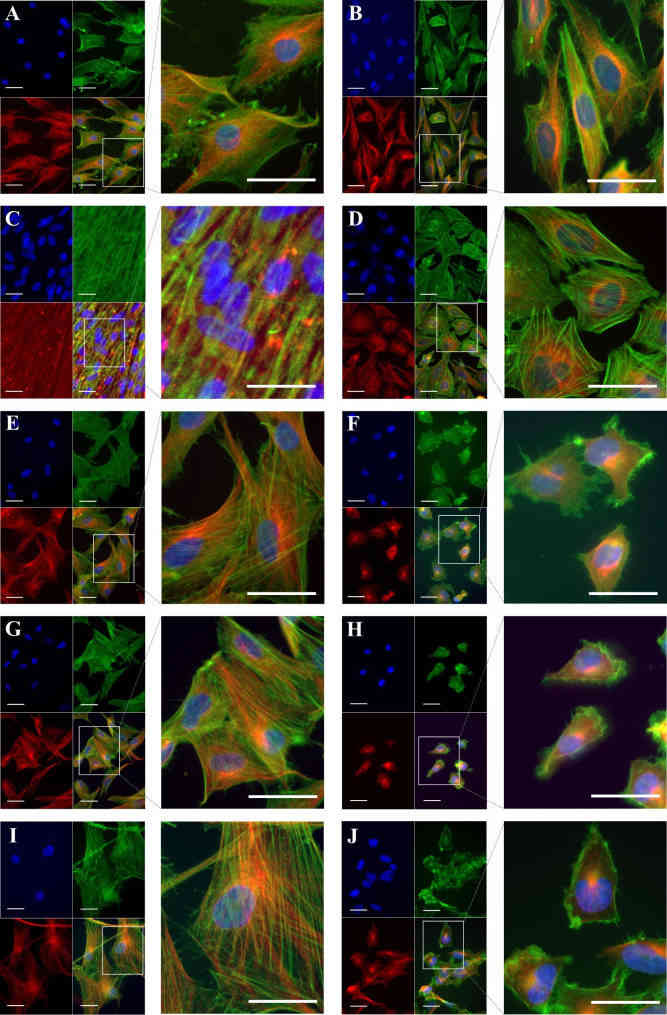 Fig. 2 Cytoskeleton
structure in musculoskeletal cancer lines and healthy cells. Healthy cells: (A) chondrocytes, (C) osteoblasts,
(E) MSC, (G) fibroblasts, (I) SKMC and their corresponding cancer cell line: (B) chondrosarcoma, (D)
osteosarcoma, (F) Ewing sarcoma, (H) fibrosarcoma and (J) rhabdomyosarcoma were subjected to β-tubulin
immunolabelling (red) coupled with fluorescence labeling of F-actin (green). (Daniel C, et al, 2023)
Fig. 2 Cytoskeleton
structure in musculoskeletal cancer lines and healthy cells. Healthy cells: (A) chondrocytes, (C) osteoblasts,
(E) MSC, (G) fibroblasts, (I) SKMC and their corresponding cancer cell line: (B) chondrosarcoma, (D)
osteosarcoma, (F) Ewing sarcoma, (H) fibrosarcoma and (J) rhabdomyosarcoma were subjected to β-tubulin
immunolabelling (red) coupled with fluorescence labeling of F-actin (green). (Daniel C, et al, 2023)
Trabectedin Improves Cytotoxic Effect of IR in STS Cells
Trabectedin is used for the treatment of advanced soft tissue sarcomas (STSs). In this study, if trabectedin could enhance the efficacy of irradiation (IR) by increasing the intrinsic cell radiosensitivity and modulating tumor micro-environment was evaluated in fibrosarcoma (HS 93.T), leiomyosarcoma (HS5.T), liposarcoma (SW872), and rhabdomyosarcoma (RD) cell lines. The IC50 of trabectedin was 1.296 nM for LMS, 0.6836 nM for LPS, 0.9654 nM for RMS, and 0.8549 nM for FS. Then, cell viability is reported as a percentage versus the control (Fig. 3).
Among all the STS cell lines, LMS and LPS showed a significant reduction of SF after trabectedin + IR compared to IR alone, both at 4 Gy and 6 Gy (Fig. 4). LMS showed the highest significant radiosensitization with an ER50 of 2.35, whereas LPS showed an ER50 of 1.45. Both FS and RMS did not show a significant difference in SF when treated with trabectedin + IR compared to the control. The observed SF in the combined treatment of trabectedin + IR in LMS and LPS cells was significantly lower than the expected SF, suggesting a synergistic effect between trabectedin and IR on clonogenic cell death. Conversely, RMS and FS showed an additive effect (Fig. 5).
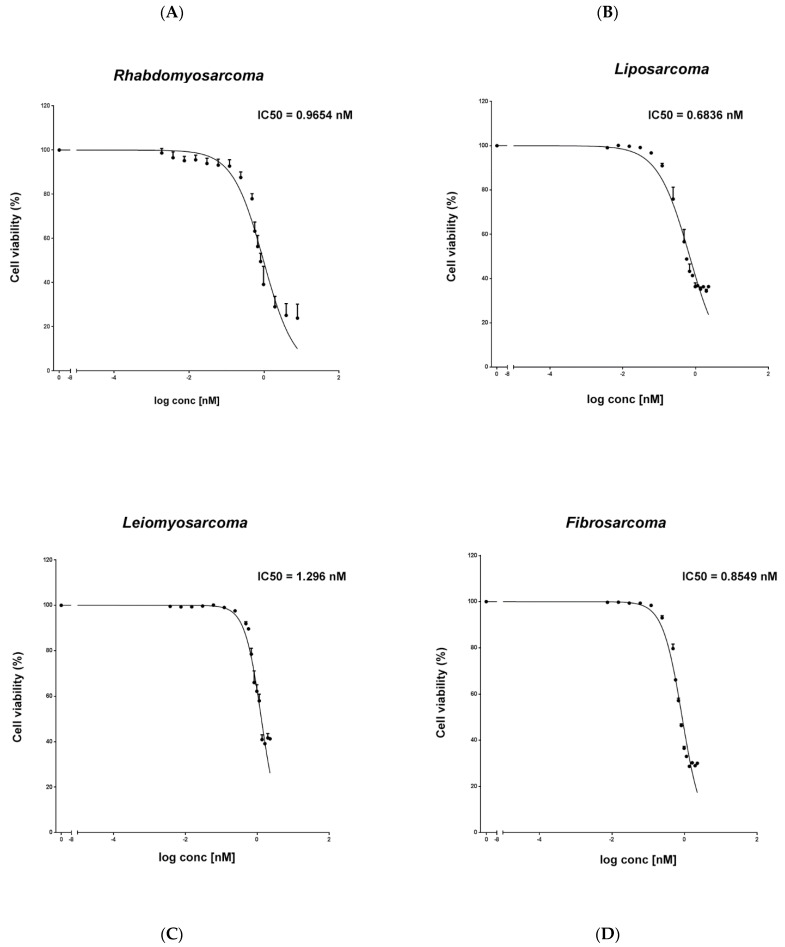 Fig. 3 Evaluation of the
cytotoxic effect of trabectedin in STS cell lines. An MTS assay was used to determine cell viability of
rhabdomyosarcoma RD (A), liposarcoma SW872 (B), leiomyosarcoma HS5.T (C), and fibrosarcoma HS 93.T (D) cells
treated with trabectedin. (Loi M, et al., 2022)
Fig. 3 Evaluation of the
cytotoxic effect of trabectedin in STS cell lines. An MTS assay was used to determine cell viability of
rhabdomyosarcoma RD (A), liposarcoma SW872 (B), leiomyosarcoma HS5.T (C), and fibrosarcoma HS 93.T (D) cells
treated with trabectedin. (Loi M, et al., 2022)
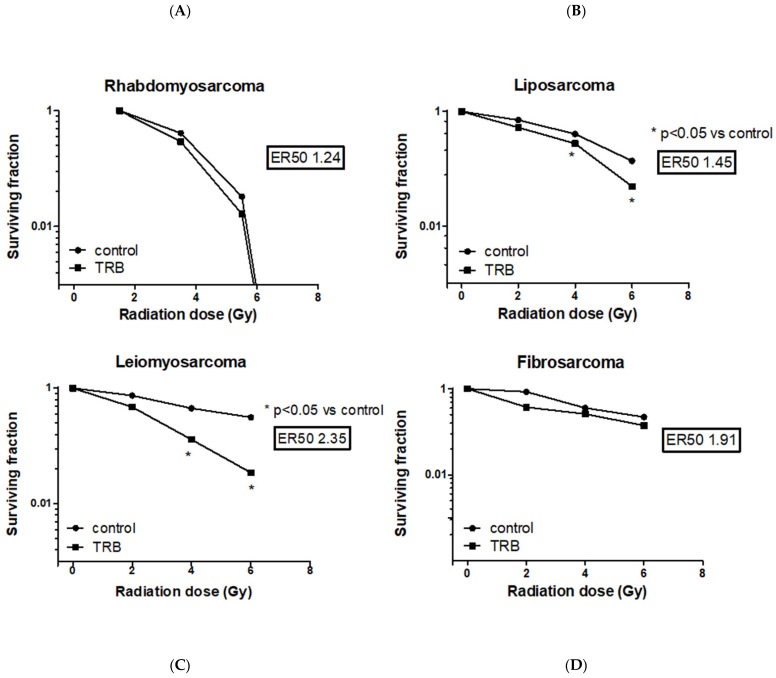 Fig.
4 Evaluation of surviving fraction after irradiation with increasing doses in rhabdomyosarcoma RD (A),
liposarcoma SW872 (B), leiomyosarcoma HS5.T (C), and fibrosarcoma HS 93.T (D) cell lines, respectively, the
control, pretreated with trabectedin, were irradiated with 0-6 Gy. (Loi M, et al., 2022)
Fig.
4 Evaluation of surviving fraction after irradiation with increasing doses in rhabdomyosarcoma RD (A),
liposarcoma SW872 (B), leiomyosarcoma HS5.T (C), and fibrosarcoma HS 93.T (D) cell lines, respectively, the
control, pretreated with trabectedin, were irradiated with 0-6 Gy. (Loi M, et al., 2022)
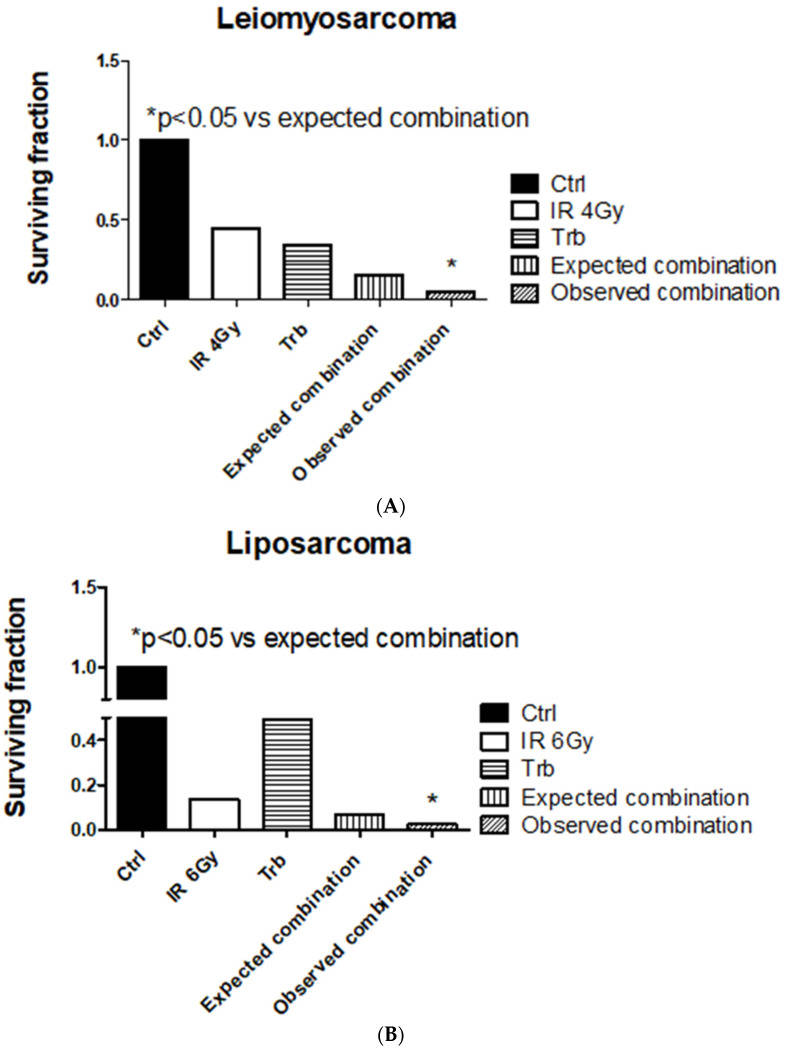 Fig.
5 Trabectedin (trb) combined with radiation-induced synergistic effect in leiomyosarcoma (HS5.T) and liposarcoma
(SW872) cells. (A) Leiomyosarcoma HS5.T. (B) Liposarcoma SW872. (Loi M, et al., 2022)
Fig.
5 Trabectedin (trb) combined with radiation-induced synergistic effect in leiomyosarcoma (HS5.T) and liposarcoma
(SW872) cells. (A) Leiomyosarcoma HS5.T. (B) Liposarcoma SW872. (Loi M, et al., 2022)
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (999)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (8)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (29)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)