Neutralization Assay Service
The outbreak of 2019 novel coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) threatens global public health. The development of vaccines capable of inducing neutralizing antibodies (NAbs) is a key goal to control the global COVID-19 pandemic.
Neutralization assay is a powerful technique to evaluate the neutralizing ability of therapeutics against coronavirus. The neutralizing ability serves as a critical indication of their efficacy for prevention and treatment of SARS-CoV-2 infections. Typically, the test substance is incubated with live virus or pseudovirus prior to the complex is applied to effector cells. This assay determines the concentration needed for 50% inhibition of infection (IC50) in vitro and enables comparison of antiviral efficacy between different treatments.
Creative Bioarray offers Neutralization Assay Service to evaluate anti-sera, antibodies, and the compound of interest against SARS-CoV-2. Our neutralization service includes Pseudotype-Based Neutralization Assay and Authentic SARS-CoV-2 Neutralization Assay.
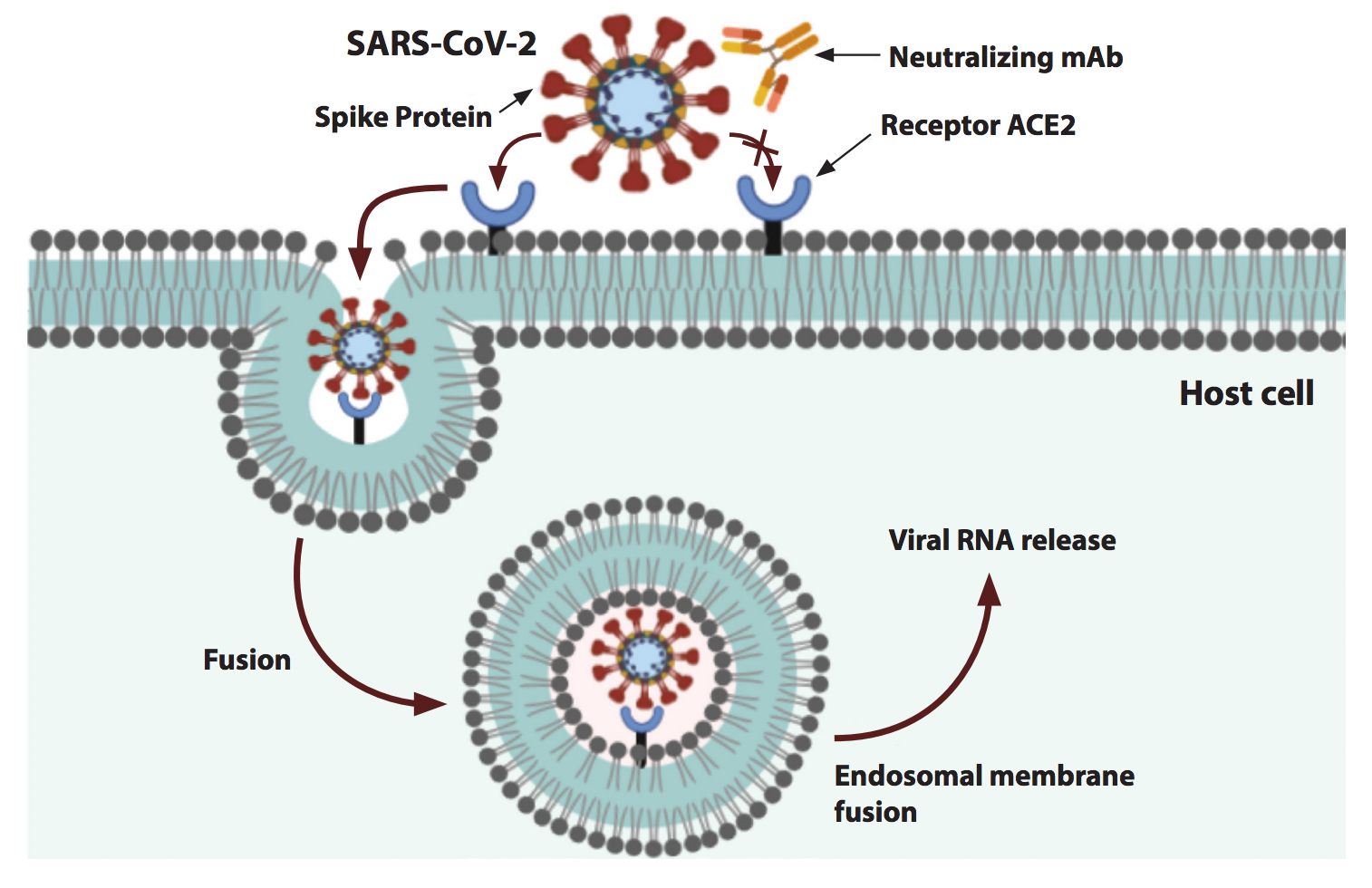 Fig.1 Competition of neutralizing antibodies with ACE2 for binding to the spike protein of SARS-CoV-2.
Fig.1 Competition of neutralizing antibodies with ACE2 for binding to the spike protein of SARS-CoV-2.
Pseudotype-Based Neutralization Assay
Pseudotyped-based neutralization assay employs pseudovirus as an alternative to wild-type SARS-CoV-2. The infectivity of pseudotyped SARS-CoV-2 is limited to a single round of replication. Thus, pseudovirus can be handled in BSL-2 facilities. The Spike (S) protein of SARS-CoV-2 binds to the human ACE2 receptor expressed on the cell surface to mediate viral entry and infection. Creative Bioarray employs SARS-CoV-2 S pseudovirus as the surrogate of replicating virus and human ACE2 stable cell lines as the target cells for pseudotype-based neutralization assay service.
Pseudotype-based method enables safe and rapid neutralization analysis to study functional antibody response against coronaviruses. This method has advantages of safety, superior sensitivity, reproductivity and high throughput capacity.
Authentic SARS-CoV-2 Neutralization Assay
To support development and validation of therapies for SARS-CoV-2, we also expand our capabilities for neutralization assay of live replicating SARS-CoV-2, which must be handled in BSL-3 laboratory. If interested, please feel free to contact us for more information. Our scientists are committed to offering the best services to satisfy your every research need.
Related Products
- Pseudoviruses
Compared with authentic SARS-CoV-2, pseudovirus is a biologically safe, reliable and effective tool for COVID-19 Studies. Pseudovirus can be processed with safety, has extensive host ranges and exhibits high competency of transfection in cells.
| Cat # | Product Name |
| CoV-001 | SARS-CoV-2 S Pseudotyped GFP Lentivirus |
| CoV-002 | SARS-CoV-2 S Pseudotyped Luciferase Lentivirus |
| CoV-009 | SARS-CoV-2 S Pseudotyped mCherry Lentivirus |
| CoV-010 | SARS-CoV-2 S-ΔG-mCherry Pseudotyped VSV |
| CoV-011 | AAV-SARS-CoV2 S-GFP |
| CoV-012 | SARS-CoV-2 S-ΔG-GFP Pseudotyped VSV |
| CoV-013 | SARS-CoV-2 S-ΔG-Luciferase Pseudotyped VSV |
| CoV-014 | SARS-CoV-2 S-ΔG Pseudotyped VSV |
| CoV-015 | SARS-CoV-2 S Pseudotyped GFP-Luciferase Lentivirus |
| CoV-016 | SARS-CoV-2 S D614G Pseudotyped GFP-Luciferase Lentivirus |
| CoV-017 | SARS-CoV-2 S D614G Pseudotyped mCherry-Luciferase Lentivirus |
- hACE2 Stable Cell Lines
Creative Bioarray offers various stable cell lines overexpressing human ACE2 genes, which have been validated and can be used for in vitro neutralization assays to screen potential NAbs or drug candidates.
| Cat # | Product Name | Description |
| CSC-RO0292 | hACE2 Stable Cell Line - HEK293 | Human Embryonic Kidney-Derived Cell Line |
| CSC-RO0641 | hACE2 Stable Cell Line - HEK293T | Human Embryonic Kidney-Derived Cell Line |
| CSC-RO0645 | hACE2 Stable Cell Line - Vero | Monkey Kidney Cell Line |
| CSC-RO0646 | hACE3 Stable Cell Line - BHK | Hamster Kidney Fibroblast Cell Line |
| CSC-RO0289 | hACE2 Stable Cell Line - HeLa | Human Cervical Cancer Cell-Derived Cell Line |
| CSC-RO0291 | hACE2 Stable Cell Line - NIH-3T3 | Mouse Embryonic Fibroblast-Derived Cell Line |
| CSC-RO0293 | hACE2 Stable Cell Line - CHO-K1 | Chinese Hamster Ovary-Derived Cell Line |
| CSC-RO0642 | hACE2 Stable Cell Line - A549 | Human Lung Carcinoma-Derived Cell Line |
| CSC-RO0290 | hACE2 Stable Cell Line - COS7 | Monkey Kidney Fibroblast-Derived Cell Line |
We'd love to hear from you
If you are interested in our neutralization service and related products, contact us today to get more information. Our scientists would be happy to answer your questions. Please let us know your research interests. We look forward to working with you.
References
- Zhou G, et al., Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci. 2020 Mar 15;16(10):1718-1723.
- Shanmugaraj B, et al., Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 38.1 (2020): 10-18.
- Wang, Chunyan, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nature Communications 11.1 (2020): 1-6.