Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Breast Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
- FAQ
Breast tumor cells, within the realm of oncology, are a critical area of study due to their complex nature and significant impact on health. These cells originate from breast tissue and can manifest in various forms, including ductal carcinoma in situ (DCIS), invasive ductal carcinoma, lobular carcinoma, and other less common subtypes. Understanding the cellular characteristics and behavior of breast tumor cells is essential for accurate diagnosis and effective treatment interventions.
Breast tumor cells proliferate abnormally within the breast tissue, forming masses or lumps that may be detected through physical examination, imaging studies, or biopsy. These cells often exhibit genetic and molecular alterations that drive their uncontrolled growth and potential spread to other parts of the body. The classification of breast tumor cells into different subtypes based on hormone receptor status (such as estrogen and progesterone receptors) and human epidermal growth factor receptor 2 (HER2) expression is crucial for tailoring treatment approaches.
Genetic and Molecular Characteristics of Breast Cancer Cells
- Oncogenes and tumor suppressor genes. Genetic mutations in oncogenes, such as HER2 (human epidermal growth factor receptor 2), and tumor suppressor genes, including TP53 and BRCA1/2, are commonly observed in breast tumor cells. These mutations can lead to dysregulated cell proliferation, impaired DNA repair mechanisms, and evasion of apoptosis, promoting tumor growth and survival.
- HER2 amplification. Amplification of the HER2 gene, leading to overexpression of HER2 protein on the surface of breast tumor cells, is associated with aggressive tumor behavior. HER2-positive breast tumors often exhibit rapid growth and a higher risk of metastasis, necessitating targeted anti-HER2 therapies such as trastuzumab and pertuzumab.
- Molecular subtypes. Breast tumor cells are classified into distinct molecular subtypes based on gene expression profiling, including luminal A, luminal B, HER2-enriched, and basal-like subtypes. Each subtype exhibits characteristic molecular features that influence treatment selection and patient prognosis.
Disease modeling and drug screening
Breast tumor cells can be used in disease modeling and drug screening processes. By establishing robust in vitro and in vivo models that accurately recapitulate the complex tumor microenvironment, researchers can investigate the underlying drivers of breast cancer progression and evaluate the efficacy of novel therapeutic agents.
Biomarker discovery and validation
The inherent heterogeneity of breast tumor cells has also made them invaluable in the field of biomarker discovery and validation. By studying the molecular signatures and phenotypic characteristics of these cells, researchers have identified a range of potential biomarkers that hold promise for early detection, disease monitoring, and treatment response prediction.
These biomarkers, which may include specific genetic mutations, protein expression patterns, or metabolic alterations, can serve as powerful tools for clinicians to guide treatment decisions and improve patient outcomes. Through rigorous validation studies using breast tumor cell-based assays, researchers have contributed to the development of novel diagnostic and prognostic tools that are transforming the landscape of breast cancer management.
Tumor immunology and immunotherapy
Leveraging breast tumor cell models, scientists have gained unprecedented insights into the mechanisms by which these cells evade and suppress the body's immune responses, as well as the strategies they employ to create immunosuppressive tumor microenvironments. This knowledge has been instrumental in the development of novel immunotherapeutic approaches, which harness the power of the immune system to recognize and eliminate breast tumor cells. Researchers have been actively involved in the testing and optimization of various immunotherapeutic agents, including checkpoint inhibitors, CAR-T cell therapies, and cancer vaccines, using breast tumor cell-based platforms.
Knockdown of hsa_circ_001783 Inhibits Breast Cancer Cells
As next-generation sequencing technologies are developing rapidly, several circular RNAs (circRNAs) have been identified as functional molecules in regulating disease progression rather than splicing by-products. The previous study has demonstrated that circRNAs can promote breast cancer cell progression under hypoxia.
The expression of hsa_circ_001783 was fine-tuned and its cellular effects on two TNBC cell lines (MDA-MB-231 and MDA-MB-468) highly expressed hsa_circ_001783. Transfection of siRNAs that specifically targeted the junction site of hsa_circ_001783 reduced its expression by 40–50% without any effect on EBLN3 or ZCCHC7 (Fig. 1a, b). Knockdown of hsa_circ_001783 significantly inhibited cell variability of MDA-MB-231 and MDA-MB-468 on day 4 after siRNA transfection (Fig. 1c). In addition, the EdU assay showed that downregulation of hsa_circ_001783 impaired the proliferation of MDA-MB-231 and MDA-MB-468, which was inconsistent with the data in CCK8 assay (Fig. 1d). Colony formation ability was also disrupted after hsa_circ_001783 knockdown (Fig. 1e). Breast cancer patients with high expression of hsa_circ_001783 suffered from more metastases (Fig. 1f). Boyden chamber coated with or without Matrigel were used to evaluate the invasion and migration ability of breast cancer cells, respectively. After the knockdown of hsa_circ_001783, the migration ability and invasion capacity of the MDA-MB-231 and MDA-MB-468 were significantly decreased (Fig. 1f, g).
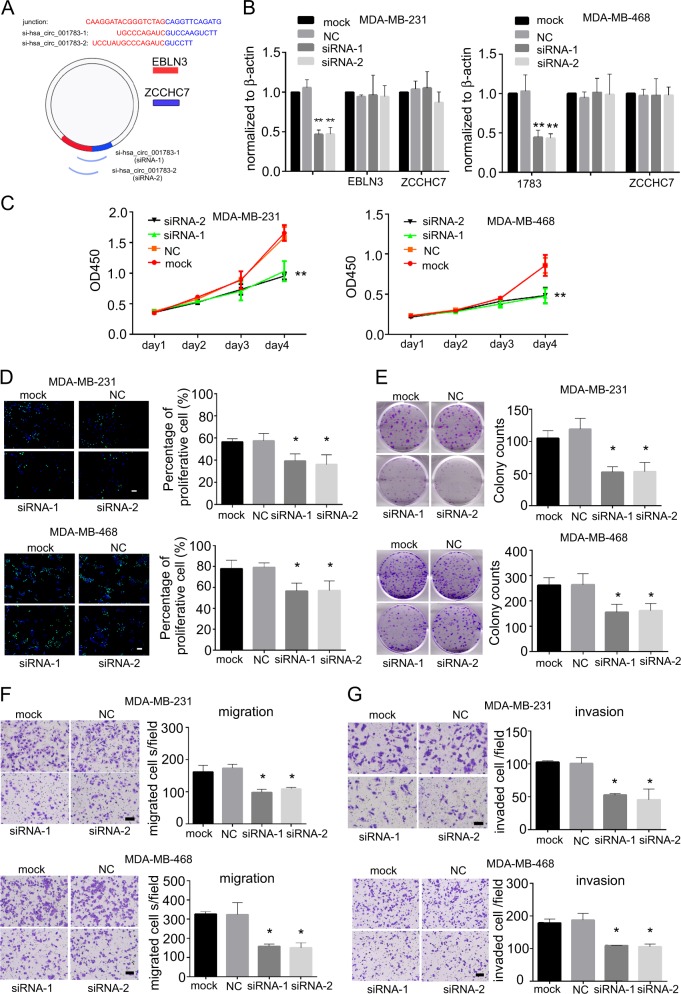 Fig. 1 Effect of hsa_circ_001783 on MDA-MB-231 and MDA-MB-468 progression. (Liu Z, et al, 2019)
Fig. 1 Effect of hsa_circ_001783 on MDA-MB-231 and MDA-MB-468 progression. (Liu Z, et al, 2019)
CPC Inhibits Human Breast Tumor Cells' Growth
1-hexadecylpyridinium (Cetylpyridinium) chloride (CPC) is a cationic surfactant composed of quaternary nitrogen bound to hydrophobic side chains. Although CPC has been consumed through mouthwashes for a long time, its antitumor and cytotoxic potential has not yet been extensively studied.
CPC was cytotoxic to both MCF-7 and MCF10A at all concentrations tested in a dose-dependent way (Fig. 2). At the lowest concentration tested (0.1 µM), cytotoxicity was over 20% f; at the highest concentration (100 µM) the cytotoxicity was 92% for MCF-7 and 77% for MCF-10A. In comparison, the cytotoxicity of the positive control, 500 µM DOX, was 86% for MCF-7 and 82% for MCF-10A. Thus, CPC had a five times higher antitumor activity than DOX. The effect of CPC on both kinds of breast human cells was dose-dependent.
To corroborate CPC cytotoxicity on breast cancer cells, LIVE/DEAD assays were carried out to verify cell death due to CPC. A 24-h exposition to 100 µM CPC depleted MCF-7 cells, while exposure to 10 µM CPC was less severe and similar to the effect of the positive cytotoxicity control of 500 µM DOX (Fig. 3). There was no cell death in drug-free MCF-7 cultures (Fig. 3; MCF-7). Altogether, these results confirm the results of the MTT assays.
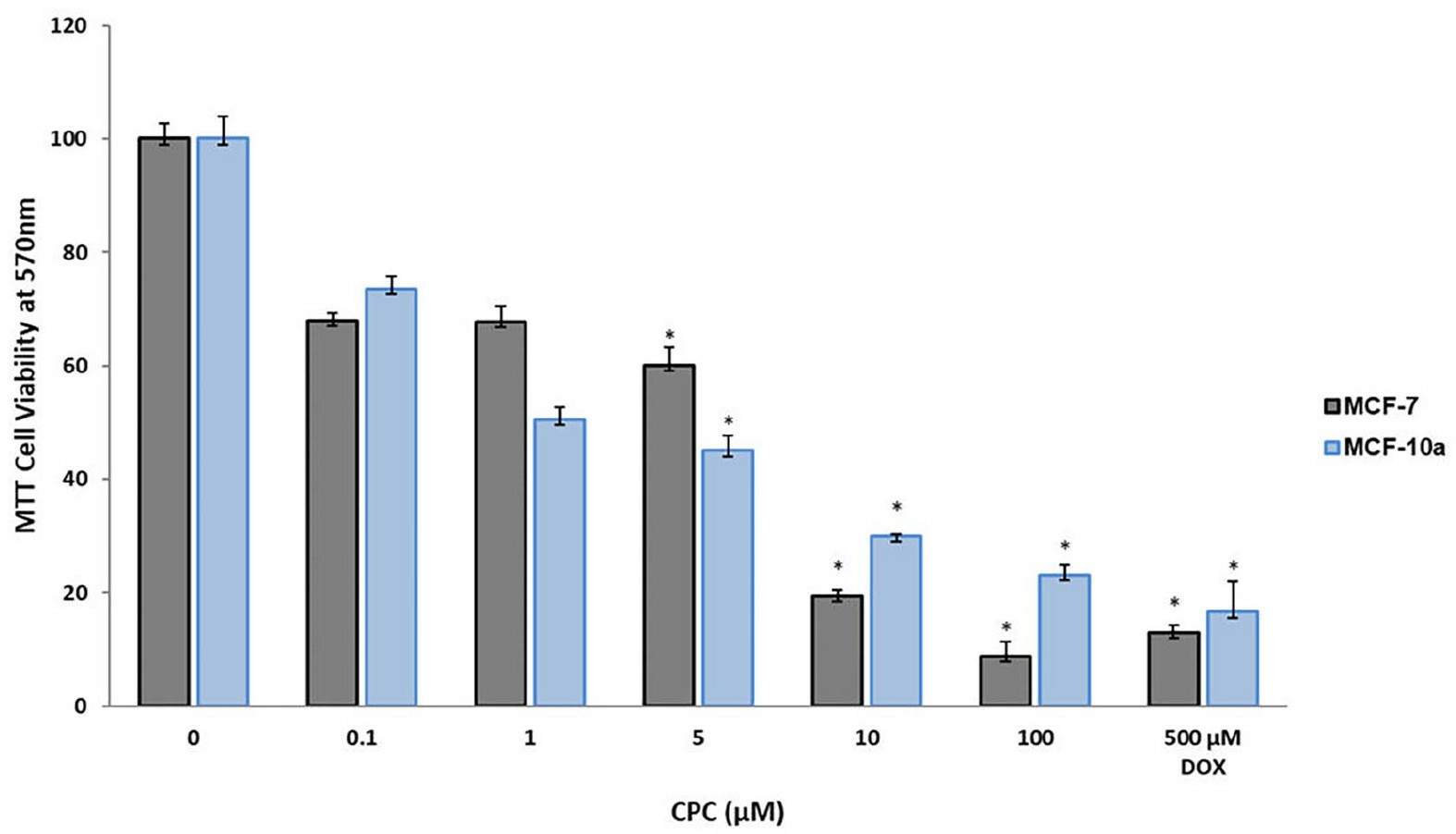 Fig. 2 Antitumor activity of CPC on human breast tumor cells. (García-Cuellar CM, et al., 2022)
Fig. 2 Antitumor activity of CPC on human breast tumor cells. (García-Cuellar CM, et al., 2022)
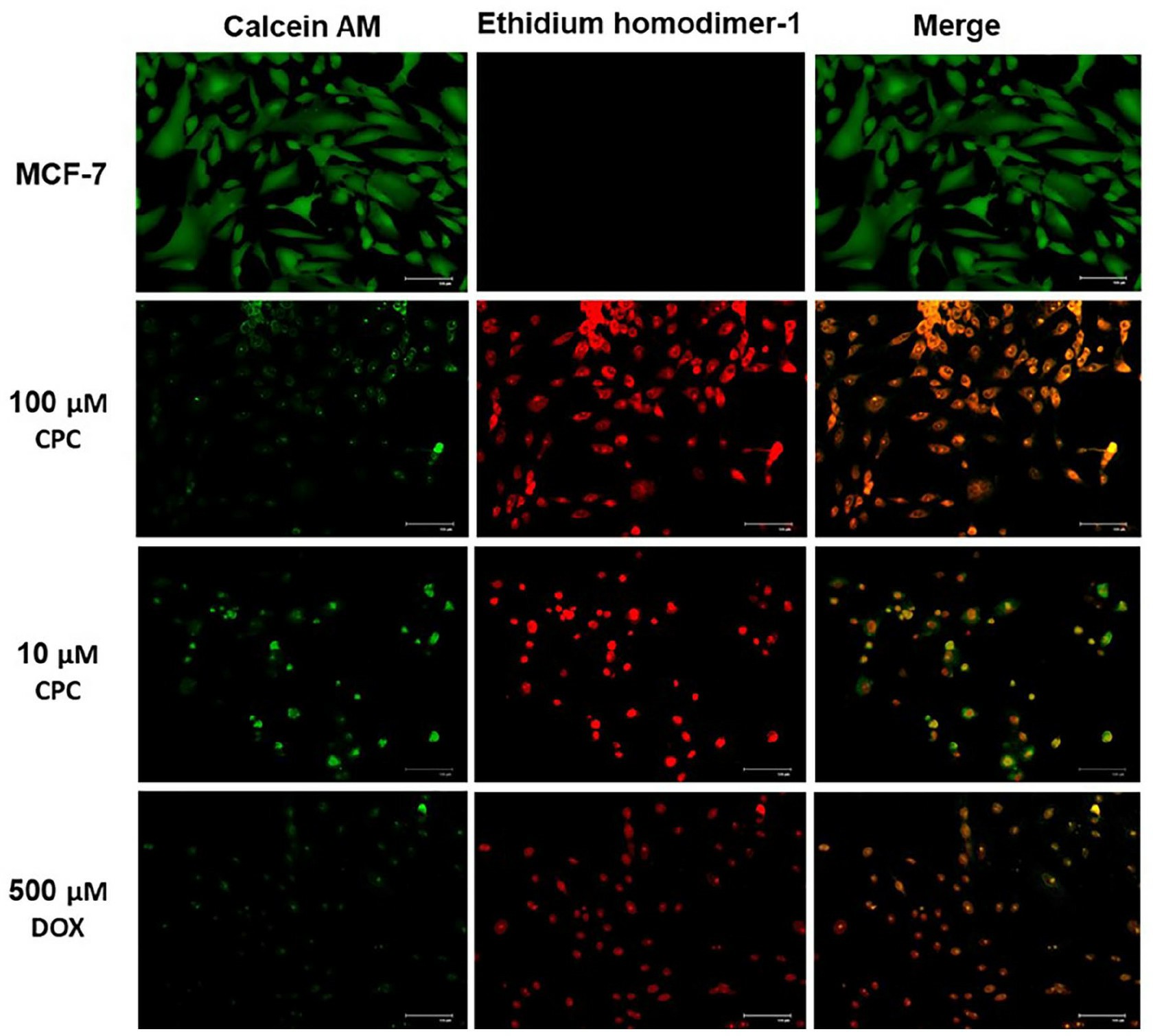 Fig. 3 LIVE/DEAD assays of CPC on MCF-7 cells. (García-Cuellar CM, et al., 2022)
Fig. 3 LIVE/DEAD assays of CPC on MCF-7 cells. (García-Cuellar CM, et al., 2022)
Different subtypes of breast cancer, such as luminal, HER2-positive, and triple-negative, are characterized by distinct genetic profiles. By studying the specific genetic alterations present in breast tumor cells, researchers have been able to identify key driver mutations and gene expression patterns that are associated with these various subtypes.
Breast tumor cells exhibit aberrant activation of signaling cascades, such as the PI3K/Akt/mTOR and MAPK pathways, which are crucial for cell proliferation, survival, and metastasis.
Breast tumor cells can exhibit a range of phenotypic and functional states, including epithelial, mesenchymal, and stem-like properties, depending on the specific cues and signals they receive from their surrounding environment. This dynamic interplay between breast tumor cells and their microenvironment has significant implications for disease progression and therapeutic response.
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Species: human, Caucasian female 69 years old;
Tissue: breast;
Tumor: adenocarcinoma...
Description: Established from the pleural effusion of a 50-year-old Caucasian woman with breast...
Description: Established from the pleural effusion of the left side from a 46-year-old Caucasian...
Description: Established from the pleural effusion metastasis of a 45-year-old woman with progressive...
Description: Established from the pleural effusion of the left side (14 days after EFM-192A) of...
Description: Established from the pleural effusion of the left side (14 days after EFM-192A) of...
Description: Established from the pleural fluid of a 50-year-old Japanese woman with ductal carcinoma...
Description: Originated from surgical material of a human breast carcinoma; the tumor was passaged...
Description: Established from the pleural effusion of a post-menopausal breast cancer patient...
Description: Established from the local metastatic cutaneous nodule of an undifferentiated carcinoma...
Description: Established from the relapsing invasive galactophoric breast adenocarcinoma resected...
Description: Established from the pleural effusion of a 43-year-old woman with metastatic breast adenocarcinoma in 1991
Description: Established from the pleural effusion from a 58-year-old French woman with terminally...

