Study of Polyploidy Leading to False Positives in FISH
Translation Lung Cancer Research. 2023 Apr 28;12 (4): 676-688.
Authors: van Gulik AL, Sluydts E, Vervoort L, Kockx M, Kortman P, Ylstra B, Finn SP, Bubendorf L, Bahce I, Sie D, Radonic T, Lissenberg-Witte B, Thunnissen E.
INTRODUCTION
In-situ hybridization (ISH) is a diagnostic tool in the detection of chromosomal anomalies, which has important implications for the diagnosis, classification, and prediction of cancer therapy in various diseases. Certain thresholds of several cells showing an aberrant pattern are commonly used to declare a sample as positive for genomic rearrangements. The phenomenon of polyploidy can be misleading in the interpretation of break-apart fluorescence in-situ hybridization (FISH). This study aims to investigate the impact of cell size and ploidy on FISH results.
METHODS
- Tissue samples. A non-small cell lung cancer (NSCLC) patient was initially diagnosed with rearrangements in both the ALK and the ROS1 gene detected by FISH analysis. Furthermore, a cohort of NSCLC cases showing polyploidy from Universitätsspital Basel and another NSCLC cohort examined by CellCarta (Antwerp, Belgium) were included in the study. As a control, liver tissue without disease was selected. All samples were neutral buffered formalin-fixed and paraffin-embedded (FFPE).
- Immunohistochemistry. Immunohistochemistry was performed on 3 µm thick tissue sections. For P80 ALK immunohistochemistry clone 5A4 was used and diluted 1/50 in Dako Diluent. Antigen retrieval was performed using a PT module high pH buffer for 20 minutes at 97°C, and staining took place in the automated staining system. For ROS1 immunohistochemistry clone D4D6 was used and diluted 1/50 in Ventana diluent.
- FISH/CISH. All sections were dried for a minimum of 60 min at 37°C. Paraffin sections were deparaffinized with xylene, rehydrated in ethanol 100%, and washed in demi water. After washing in Dako wash buffer, slides were pre-treated with 2-(N-morpholino) ethane sulfonic acid (MES) buffer at 100°C for 10 min, and treated with pepsin at 37°C. After washing, sections were dehydrated with ethanol 100% for 1 min and dried at room temperature (RT). Following denaturation (5 min at 82°C), hybridization was performed overnight (minimum of 20 h at 40°C). Post-hybridization washes were performed in 0.1 ×SSC/0.1% NP-40. Subsequently, sections were dehydrated with ethanol 100% and dried at RT. Counterstaining and mounting were performed manually with 4',6-diamidino-2-phenylindole (DAPI; 0.8 µg/mL).
- Quantitative analysis. Signal counting is conducted primarily under the fluorescence microscope to avoid artifacts introduced by image capture and the single 3' and/or split signals are considered positive for break apart.
- Copy number profiling. Copy number aberration profiling was performed with shallow sequencing and DNA isolated from FFPE samples.
- Browse our recommendations
As an established leader in the field of cell science, Creative Bioarray offers a range of high-quality products and services for our customers' research, including but not limited to the products in the table below.
| Product/Service Types | Description | Recommended Products |
| Tissue sample | A range of normal and diseased tissue blocks are provided. Our high-quality tissues are banked under strict collection protocols. | Lung cancer, non-small cell (NCSLC) FFPE tissue block, Lung small cell carcinoma FFPE tissue block, Sample Collection Service… |
| Immunohistochemistry | IHC, by exploiting the principle of antibodies binding specifically to antigens in biological tissues, is a routine method to detect antigens in cells of a tissue section. | Immunohistochemistry (IHC), Immunofluorescence (IF) Service… |
| FISH | It is a cytogenetic technique using fluorescent probes to bind the chromosome with a high degree of complementarity. | Fluorescent In Situ Hybridization (FISH) Service, Custom Probes, FFPE FISH Pretreatment Kit… |
| Molecular Karyotyping (aCGH) Service | Array Comparative Genomic Hybridization (aCGH) is a high-resolution karyotype analysis solution. | Molecular Karyotyping (aCGH) Service |
RESULTS
- Nuclear size was measured and the number of red signals (CH7) in MET CISH and double signals (fusion signals) in ALK FISH was manually counted in control liver sections of varying section thickness (2 µm and 4 µm/5 µm). In MET CISH as well as in ALK FISH the number of the signals of interest positively correlated (ρ≥0.42, all P<0.001) with nuclear size. Overall, irrespective of the ISH method used, the number of signals increases with nuclear size related to physiological polyploidy and is also related to section thickness.
- To further explore the relationship between nuclear size and several double signals (fusion signals), a pulmonary carcinoid tumor with a prominent variation in nuclear size was examined with ALK FISH and ROS1 FISH. The positive correlation (ρ≥0.73, all P<0.001) between nuclear size and ploidy in liver cells was confirmed in the carcinoid cell nuclei. To establish the relationship between single and double signals in polyploidy or high aneuploidy cases, 9 additional NSCLC cases were counted from digital ALK FISH images. Thus, larger tumor cell nuclei have a higher polyploidy/high aneuploidy rate and a higher fraction of single signals.
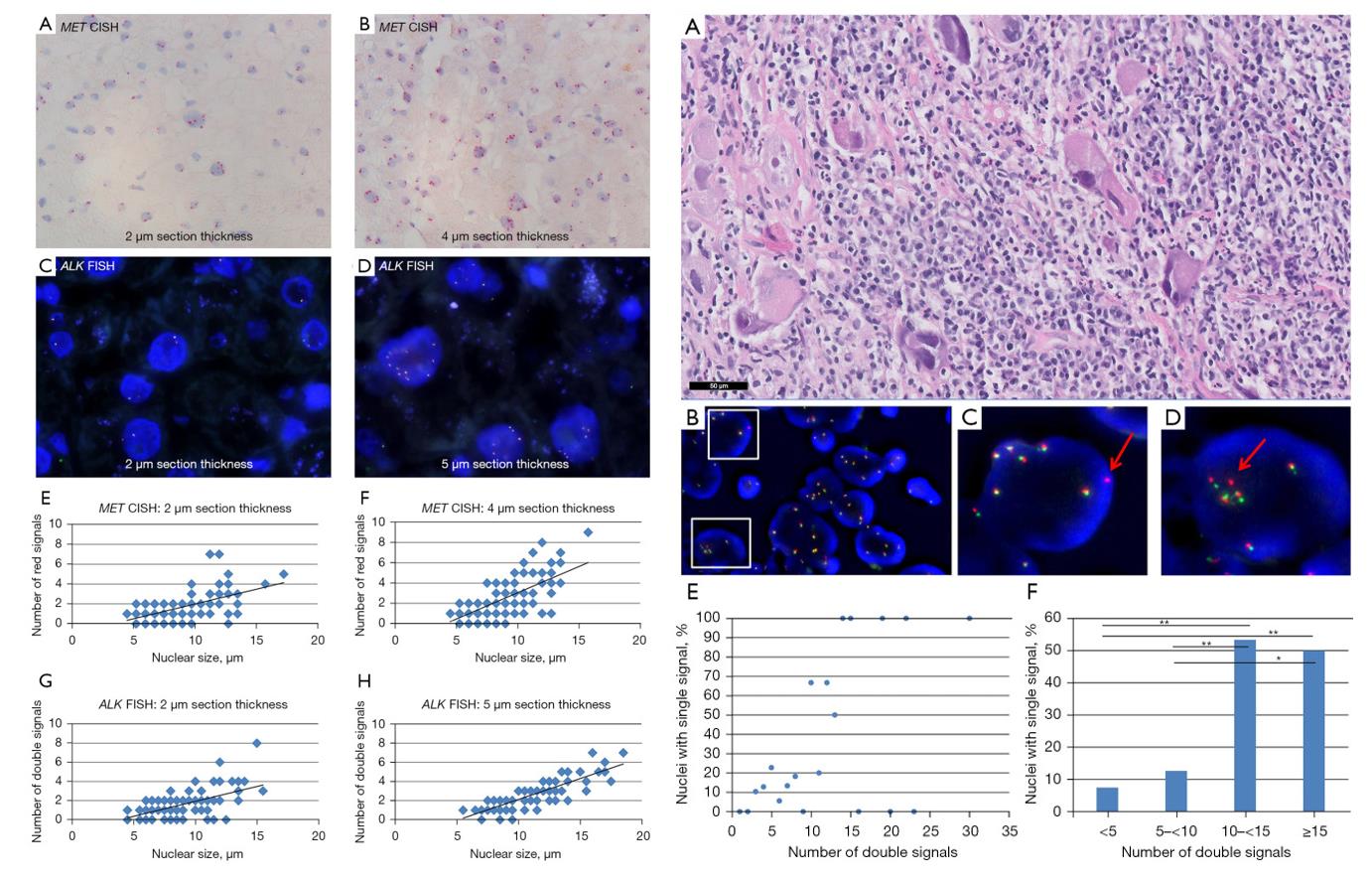 Fig. 1 Left: Representative images of ISH signals in liver control tissue containing physiologic polyploidy; Right: Single signals in polysomic tumor cell nuclei, images, and quantification.
Fig. 1 Left: Representative images of ISH signals in liver control tissue containing physiologic polyploidy; Right: Single signals in polysomic tumor cell nuclei, images, and quantification.
- Out of a total of 148 NSCLC samples with known IHC and FISH results, ten cases showed borderline ALK FISH results (FISH positivity of 15-20%). For nine out of these ten cases, discrepant results for ALK IHC and FISH testing were observed.
SUMMARY
In the case of polyploidy, there is an increased likelihood of false positivity when using break-apart FISH probes. Therefore, we state that prescribing one single cut-off in FISH is inappropriate. In polyploidy, the currently proposed cut-off should only be used with caution and the result should be confirmed by an additional technique.
RELATED PRODUCTS & SERVICES
Reference
- van Gulik AL, et al. (2023). "False positivity in break apart fluorescence in-situ hybridization due to polyploidy." Transl Lung Cancer Res. 12 (4), 676-688.