AAV Biodistribution Analysis (RNA ISH)
AAV Biodistribution Analysis (RNA ISH) Services from Creative Bioarray provide an unparalleled sensitive and specific method for cell and tissue-specific assessment of gene therapy AAV vector and transgene mRNA expression analysis in any tissue.
FDA strongly recommends biodistribution studies to characterize engineered products. RNA in situ hybridization assay is the most powerful spatial method available within the methods provided. Creative Bioarray offers Custom RNA ISH assay and ACD Bio's RNAscope ISH assay services.
As stated by the USFDA, "The biodistribution data, coupled with other preclinical safety endpoints such as clinical pathology and histopathology, help determine whether vector presence or gene expression correlates with any tissue-specific detrimental effects in animals." (FDA Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products, November 2013). This is particularly important in germ-line tissues (i.e., gonads) to determine intertied transmission status. Our AAV Biodistribution Analysis (RNA ISH) service can help you validate and accelerate your gene therapy development.
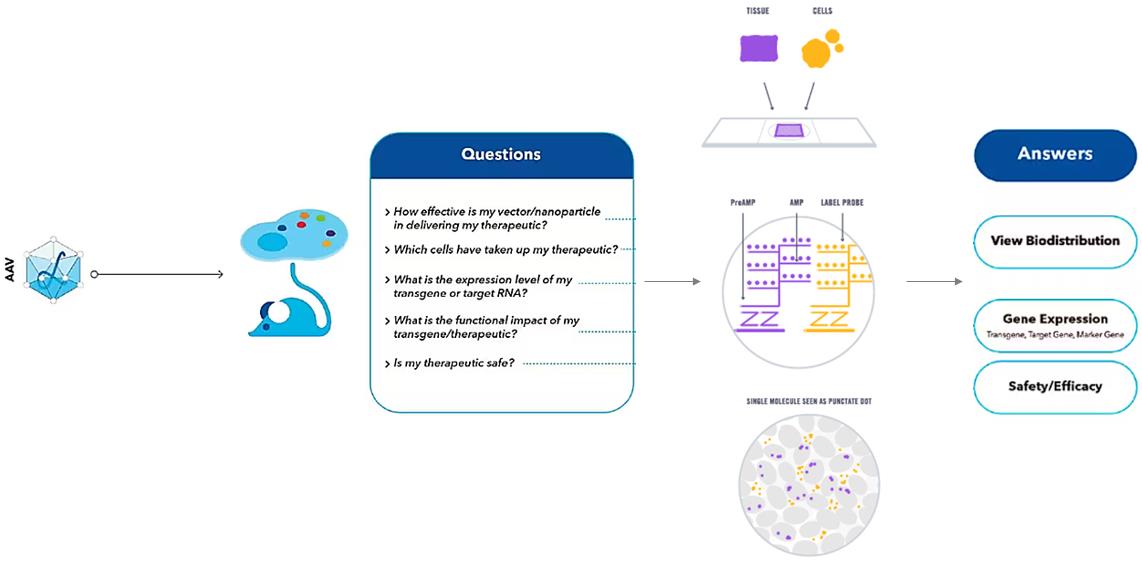 Figure 1. RNA ISH provides information about AAV Biodistribution.
Figure 1. RNA ISH provides information about AAV Biodistribution.
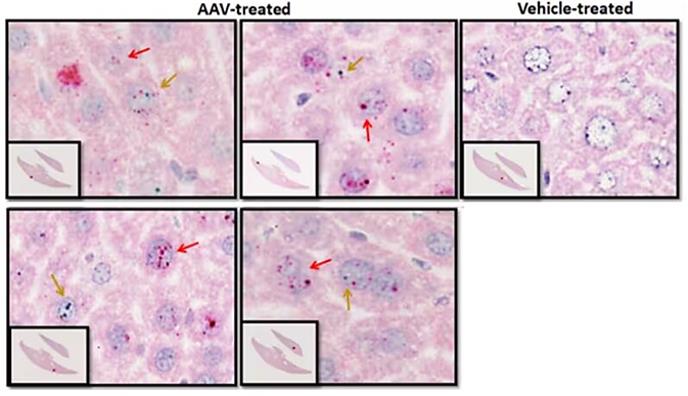 Figure 2. RNAscope probe staining allows visualization and quantification of transgene expression (red arrow) and AAV vector (green arrow) presence in treated liver samples.
Figure 2. RNAscope probe staining allows visualization and quantification of transgene expression (red arrow) and AAV vector (green arrow) presence in treated liver samples.
The RNA ISH is an excellent solution for detecting AAV vector DNA and therapeutic transgene mRNA expression within intact tissue morphology, addressing critical questions on tissue biodistribution, persistence, cellular tropism, and vector promoter activity. As a leading technology provider, Creative Bioarray's elite scientists have broad experience in AAV Biodistribution Analysis (RNA ISH), which can supply the best service for you.
Advantages of RNA ISH over qPCR-based methods for AAV Biodistribution Analysis:
- Morphology-Based Quantification: Instead of providing average values, RNA ISH gives detailed information about the morphology-based distribution.
- Enhanced Accuracy: RNA ISH can potentially provide more accurate biodistribution details in the field of gene-editing therapies than qPCR methods.
- Comprehensive Analysis: RNA ISH not only quantifies nucleic acids but also allows for a more thorough analysis of their morphological distribution, adding an extra layer of information for researchers.
- Detailed Visualization: RNA ISH enables the visualization of the spatial distribution of the target molecule, which is not possible with qPCR.
- In-Situ Analysis: RNA ISH offers in-situ analysis, meaning that it can provide information about the specific location of gene expression within a specimen. qPCR, on the other hand, only provides data based on bulk analysis.
Benefits of AAV Biodistribution Analysis (RNA ISH)
- Simultaneously visualize in vivo delivery of vector DNA and therapeutic transgene mRNA
- Identify cell tropism of AAV vector by multiplexing with cell-type markers
- Distinguish transgene from endogenous sequences, at the single nucleotide level
- Quantify AAV+ cell number and track persistence over time
Creative Bioarray offers AAV Biodistribution Analysis (RNA ISH) for you as follows:
- Probe design
- Probe synthesis
- ISH staining
- Imaging
- Data analysis
Quotation and ordering
Our customer service representatives are available 24hr a day! We thank you for considering Creative Bioarray as your AAV Biodistribution Analysis (RNA ISH) partner.
References
- EMEA. (2009) General principles to address virus and vector shedding, in ICH Considerations, EMEA/CHMP/ICH/449035/442009.
- EMEA. (1997) Preclinical safety evaluation of biotechnologicy-derived pharmaceuticals S6, in ICH harmonised tripartite guideline.
- EMEA. (2009) Addenedum to ICH S6: Preclinical safety evaluation of biotechnologicy-derived pharmaceuticals S6(R1), in ICH draft consensus guideline.
- Schenk-Braat, E. A., van Mierlo, M. M., Wagemaker, G., Bangma, C. H., and Kaptein, L. C. (2007) An inventory of shedding data from clinical gene therapy trials, J Gene Med 9, 910–921.
- Stieger, K., Schroeder, J., Provost, N., Mendes-Madeira, A., Belbellaa, B., Le Meur, G., Weber, M., Deschamps, J. Y., Lorenz, B., Moullier, P., and Rolling, F. (2009) Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates, Mol Ther 17, 516–523.
- Manno, C. S., Pierce, G. F., Arruda, V. R., Glader, B., Ragni, M., Rasko, J. J., Ozelo, M. C., Hoots, K., Blatt, P., Konkle, B., Dake, M., Kaye, R., Razavi, M., Zajko, A., Zehnder, J., Rustagi, P. K., Nakai, H., Chew, A., Leonard, D., Wright, J. F., Lessard, R. R., Sommer, J. M., Tigges, M., Sabatino, D., Luk, A., Jiang, H., Mingozzi, F., Couto, L., Ertl, H. C., High, K. A., and Kay, M. A. (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response, Nat Med 12, 342–347.
- Toromanoff, A., Cherel, Y., Guilbaud, M., Penaud-Budloo, M., Snyder, R. O., Haskins, M. E., Deschamps, J. Y., Guigand, L., Podevin, G., Arruda, V. R., High, K. A., Stedman, H. H., Rolling, F., Anegon, I., Moullier, P., and Le Guiner, C. (2008) Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle, Mol Ther 16, 1291–1299.

