In Vitro Genotoxicity
Creative Bioarray offers a series of genotoxicity tests to identify chemicals that can cause genetic alterations in cells following the OECD test guidelines, including bacterial reverse mutation test OECD 471 (also known as Ames test), in vitro mammalian chromosomal aberration test OECD 473, in vitro mammalian cell micronucleus test OECD 487, in vitro gene mutation assays OECD 490, as well as other genotoxicity assays such as in vitro comet assay and genotoxicity screening.
Genotoxicity, compared to other types of toxicity, may result in severe consequences that can be amplified and inherited after exceptionally long periods following exposure. Even DNA damage that occurs in a single cell caused by genotoxic drugs at low exposure can cause unexpected severe consequences in the long run.
Genotoxicity includes mutagenicity that involves alterations in the DNA structure, as well as DNA damage that may not result in permanent alterations in the DNA structure. Therefore, genotoxicity studies include tests that detect gene mutations, structural and numerical chromosomal aberrations and abnormalities, as well as tests that evaluates induced DNA damages resulted from DNA strand breaks and DNA adducts.
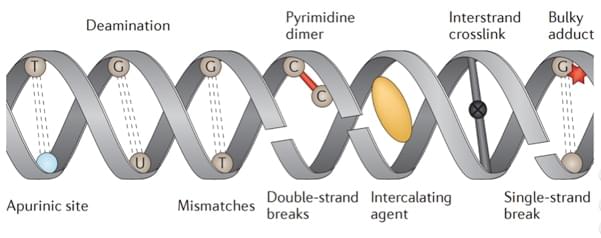 Fig 1. An overview of types of DNA damage
Fig 1. An overview of types of DNA damage
In order to identify genotoxic chemicals early in the drug development process, Creative Bioarray has developed a variety of in vitro genotoxicity tests, including but not limited to:
| • | Bacterial reverse mutation test OECD 471 |
| • | In vitro mammalian chromosomal aberration test OECD 473 |
| • | In vitro mammalian cell micronucleus test OECD 487 |
| • | In vitro gene mutation tests OECD 490 |
| • | In vitro comet assay |
| • | Genotoxicity screening |
Creative Bioarray, having gained deep knowledge and experience in toxicological research and compound safety evaluation throughout the years, is the first-choice partner for consulting and performing in vitro genotoxicity assays. We also provide in vitro toxicity assays on other tissues and organs. If you have any special needs or questions regarding our services, please feel free to contact us to get support from our experienced experts. We look forward to working with you in the near future.
References
- Helleday, Thomas, Saeed Eshtad, and Serena Nik-Zainal. "Mechanisms underlying mutational signatures in human cancers." Nature Reviews Genetics 15.9 (2014): 585-598.
- Žegura, Bojana, and Metka Filipič. Application of in vitro comet assay for genotoxicity testing. Humana Press, 2004.

